SalivaDirect™ COVID-19 Testing Process < Pathology
4.6 (421) · € 25.00 · En Stock
Our quick and affordable saliva-based COVID-19 test developed by Yale scientists has received FDA Emergency Use Authorization. The Pathology Clinical Molecular

What Is the COVID-19 SalivaDirect Test?

Diagnostic performances of common nucleic acid tests for SARS-CoV-2 in hospitals and clinics: a systematic review and meta-analysis - The Lancet Microbe

SalivaDirect, Inc. (@saliva_direct) / X

SalivaDirect, Inc. (@saliva_direct) / X

SalivaDirect: A simplified and flexible platform to enhance SARS-CoV-2 testing capacity - ScienceDirect
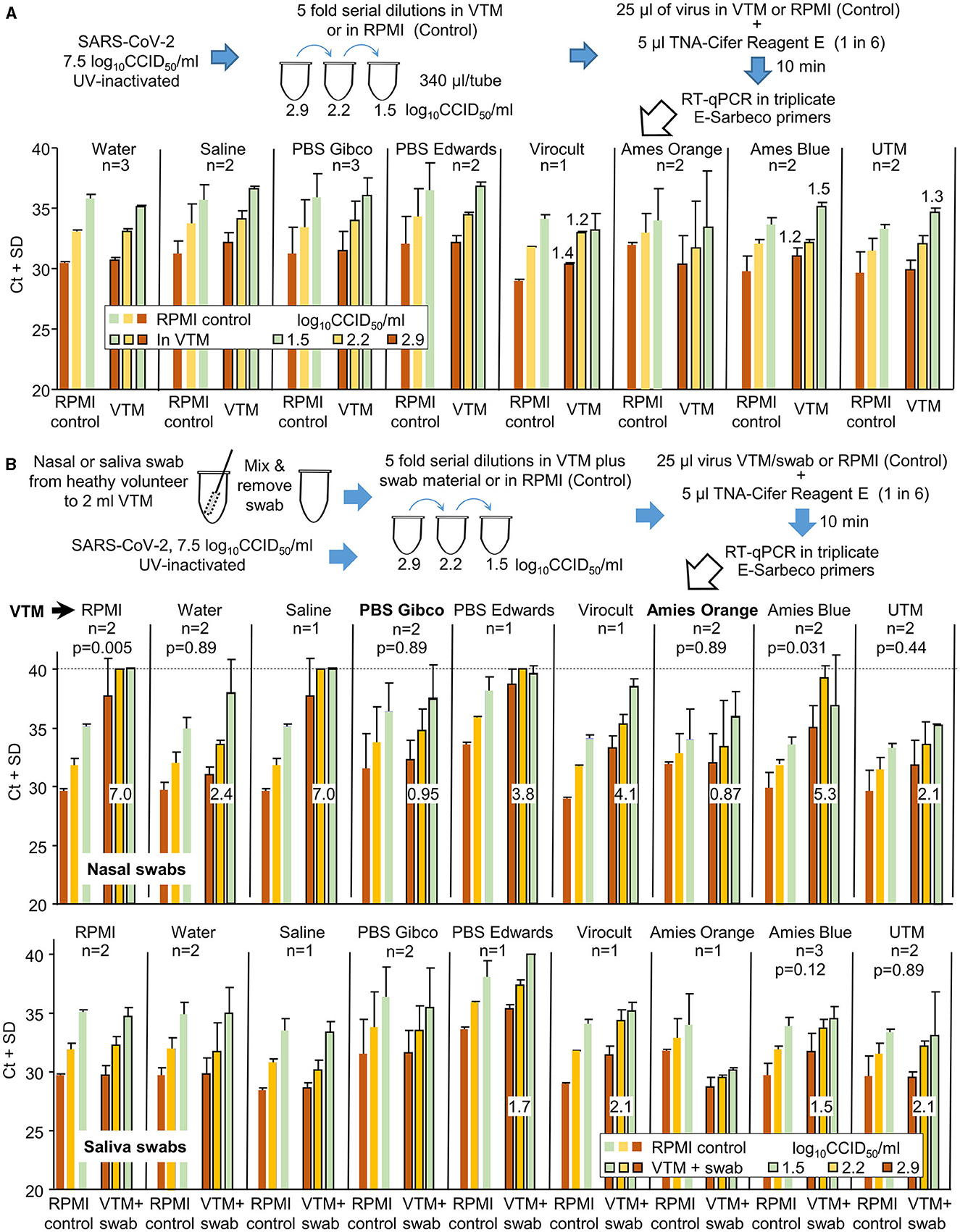
Frontiers Rapid inactivation and sample preparation for SARS-CoV-2 PCR-based diagnostics using TNA-Cifer Reagent E

SaliVISION: a rapid saliva-based COVID-19 screening and diagnostic test with high sensitivity and specificity

News SalivaDirect™

News SalivaDirect™
Low saliva pH can yield false positives results in simple RT-LAMP-based SARS-CoV-2 diagnostic tests
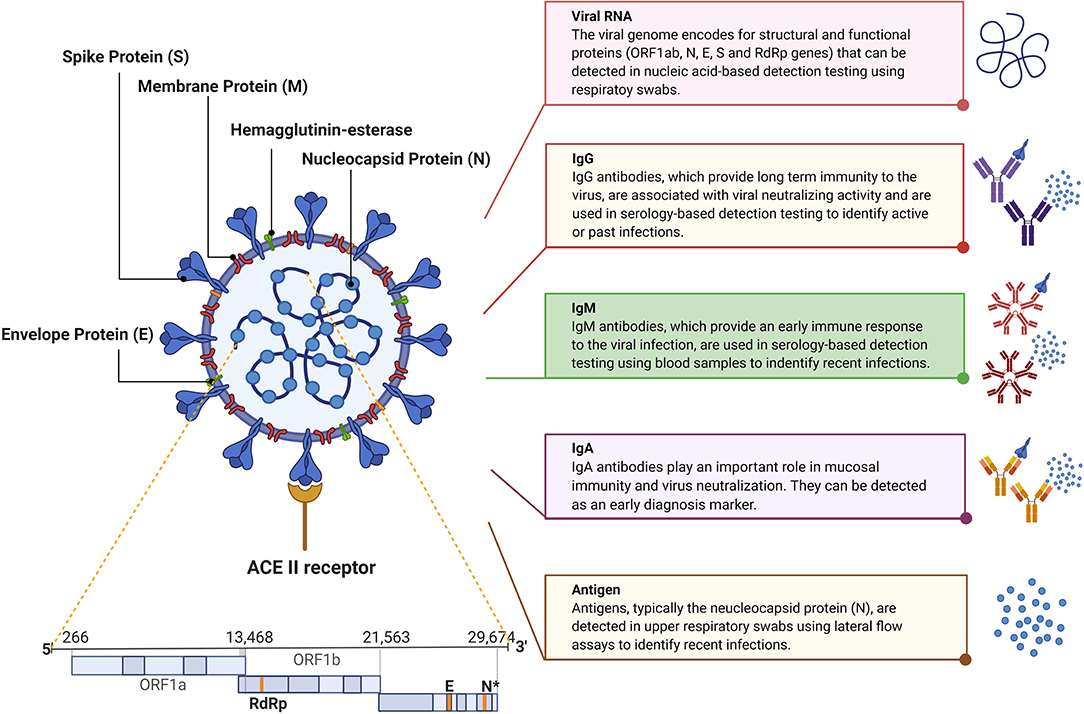
Frontiers COVID-19 in-vitro Diagnostics: State-of-the-Art and Challenges for Rapid, Scalable, and High-Accuracy Screening

SalivaDirect: A simplified and flexible platform to enhance SARS-CoV-2 testing capacity - ScienceDirect

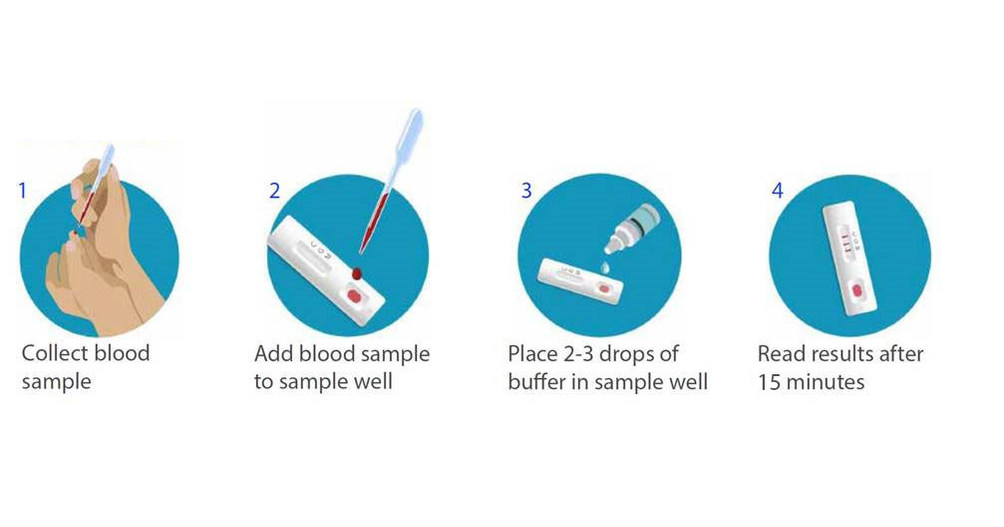
:max_bytes(150000):strip_icc()/combo-rapid-test_04-91f208ea8a2e49dd967da02768978cad.jpg)