- Accueil
- g heat
- Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is
Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is
4.9 (654) · € 18.50 · En Stock
Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is
Heat of reaction for- CO-g- - 1-2 O2-g- CO2-g-at constant V is-67-71 K cal at 17- C- The heat of reaction at constant P at 17- C is

Chapter 13 Chemical Kinetics - ppt download

heat evolved in the reaction H2 + cl2 gives to HCL is 182 kj/mole bond energy of H2,cl2 are 430 and

Activation of C−H Bonds by Metal Complexes

Heat of reaction . CO(g) + 1/O2(g) → CO2(g) constant V is-67.71 K 17℃ The heat of reaction constant P 17°C is (1-68 K a 2. 1S- (91

2. Heat of reaction , COCO) + 0.1) - COX) constant V is -67.71 Kcal 17°C. The heat of reaction constant Pat 17°C is :- (1)-68.0 Kcal (2) + 68.0 Kcal (3) - 67.42 Kcal (4) None The reaction
Heat of reaction for, CO(g)+1/2O2( g)→CO2( g) at constant V is −67.71 K..

Heat of reaction CO(g) + 12 O, (g) → CO. (g) constant Vis -67.71 Kcal 17°C. The heat of re. constant Pat 17°C is :- (1) -68.0 K (2) + 68.0 K (
Essential Pharma Documents: February 2017
(174).jpg)
Thermodynamics Exam For 12th Grade! Quiz - Trivia & Questions
Heat of reaction for, CO(g)+1/2O2( g)→CO2( g) at constant V is −67.71 K..
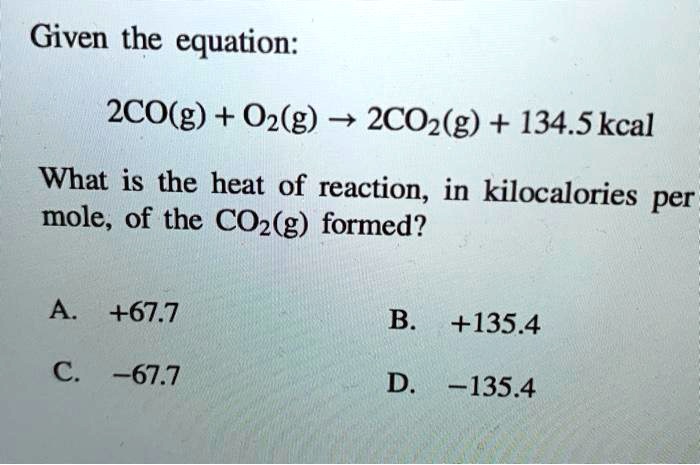
SOLVED: Given the equation: 2CO(g) + O2(g) â†' 2CO2(g) + 134.5 kcal What is the heat of reaction, in kilocalories per mole, of the CO2(g) formed? A) +67.7 B) +135.4 C) -67.7

Heat of reaction for; CO(g) + 1/2O2(g)→CO2(g) at constant V is - 67.71 cal 17^oC . The heat of reaction at constant P at 17^oC

Solved Question 27 (1 point) In the reaction, CO (g) + NO2











