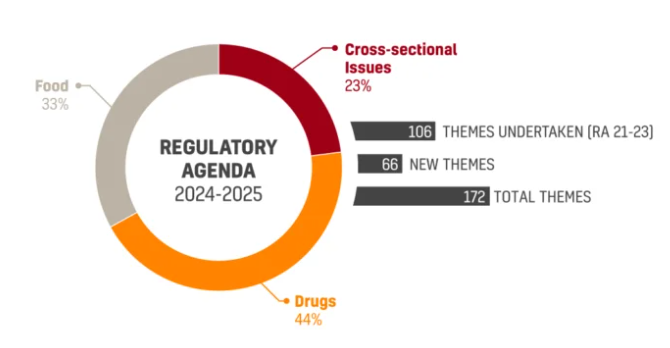
2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA
4.5 (316) · € 24.99 · En Stock
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1…

Global Service Providers Guide 2023 by Chemical Watch - Issuu

Announcements ALTEX - Alternatives to animal experimentation

SEC Filing - Alvotech

SEC Filing Alkermes plc

2021 Q2 Results Presentation & Transcript

NOVARTIS AG Form 20-F Filed 2021-01-26

D155 Board Information Packet - June 2022 - Flipbook by District 155 Technology Department

Aprovada a agenda regulatória 2024-2025 da ANVISA

Inline XBRL Viewer

Blog - Medriva

Marcelo Brisolla on LinkedIn: I recommend this training to all companies that want to do business with…
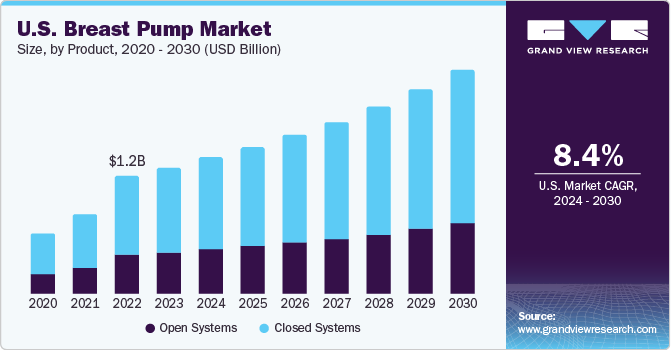
Breast Pump Market Size, Share And Trends Report, 2030

How to Prepare for and Make the Most Out of your FDA Pre-Submission: Leverage This Under-Utilized Tool to Help De-Risk your 510(k)

Federal Register :: Medicare Program; Contract Year 2025 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, and Programs of All-Inclusive Care for