- Accueil
- mcv4
- Sanofi Pasteur Announces FDA Approval of Menactra Meningococcal Conjugate Vaccine Indication for Infants
Sanofi Pasteur Announces FDA Approval of Menactra Meningococcal Conjugate Vaccine Indication for Infants
4.8 (557) · € 30.99 · En Stock
/PRNewswire/ -- Sanofi Pasteur, the vaccines division of the sanofi-aventis Group (EURONEXT: SAN and NYSE: SNY), announced today that the U.S. Food and Drug

Immunogenicity and Safety of a Meningococcal Quadrivalent Conjugate Vaccine in Saudi Arabian Adolescents Previously Vaccinated with One Dose of Bivalent and Quadrivalent Meningococcal Polysaccharide Vaccines: a Phase III, Controlled, Randomized, and
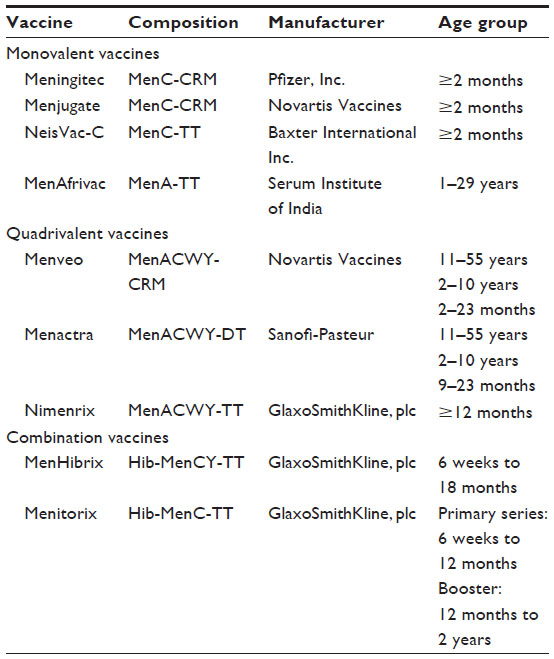
Meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate v

Meningococcal Vaccines Market Size, Share, Growth Analysis, By Type, End-User - Industry Forecast 2023-2030

PDF) Safety and Immunogenicity of a Meningococcal Quadrivalent Conjugate Vaccine in Five- to Eight-Year-Old Saudi Arabian Children Previously Vaccinated with Two Doses of a Meningococcal Quadrivalent Polysaccharide Vaccine

PDF] Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020
Menactra - Meningococcal Vaccine - Clinical Trials Arena

Meningococcal ACWYX Conjugate Vaccine in 2-to-29-Year-Olds in Mali and Gambia

Immunization Update - Advances in Pediatrics

Sanofi Ally Shantha Begins Rotavirus Vaccine Phase 3 Trial
Meningitis Vaccine Liquid at Best Price in Surat, Gujarat

Menactra® Meningitis Vaccine Meningococcal (Groups A,C,Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine, Preservative Free 4 mcg / 0.5 mL Injection Single Dose Vial 0.5 mL – Caring Med

Meningococcal Vaccine: Most Up-to-Date Encyclopedia, News & Reviews

Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance












