2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA - Lexology
4.7 (512) · € 21.99 · En Stock
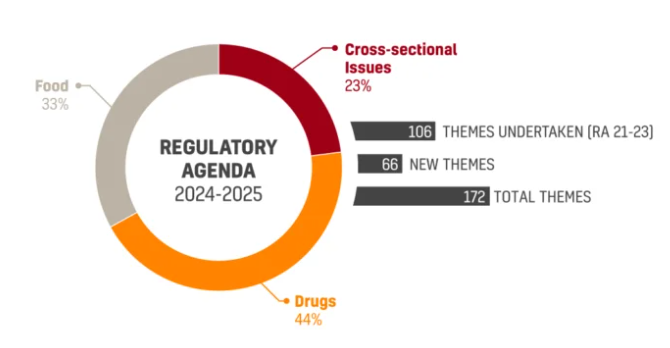
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1…

FDA Modernization Act Allows More Effective Drug Development

BioCentriq Speaking at PDA/FDA Joint Regulatory Conference
CDER Drug Safety Labeling Changes - 1/5/2024 - US FDA

New FDA Approved Drugs & Devices to Watch for in 2024

EY FICCI Indian Pharma Report 2021 I Future Is Now

American Psychoanalytic Association (APsA) 2024 National Meeting

Digital Health: Fundamentals of FDA Regulation (January 2024)

Relatório de gestão de 2022 do inpi: panorama de patentes - Lexology

Advertising law, year in review

FDA Regulatory News and Trends - January 19, 2024 - Lexology

Abstract Submissions - WORLDSymposium