CMV R-GENE® - Diagnostic Clinique
5 (482) · € 38.99 · En Stock
Quantification précise de la charge virale du CMV sur une gamme de linéarité étendue Réactifs prêts à l’emploi comprenant le contrôle interne et les standards de quantification Marquée CE-IVD sur l’ensemble des principales plateformes d’extraction et des systèmes de PCR en temps réel et sur différents types d’échantillons
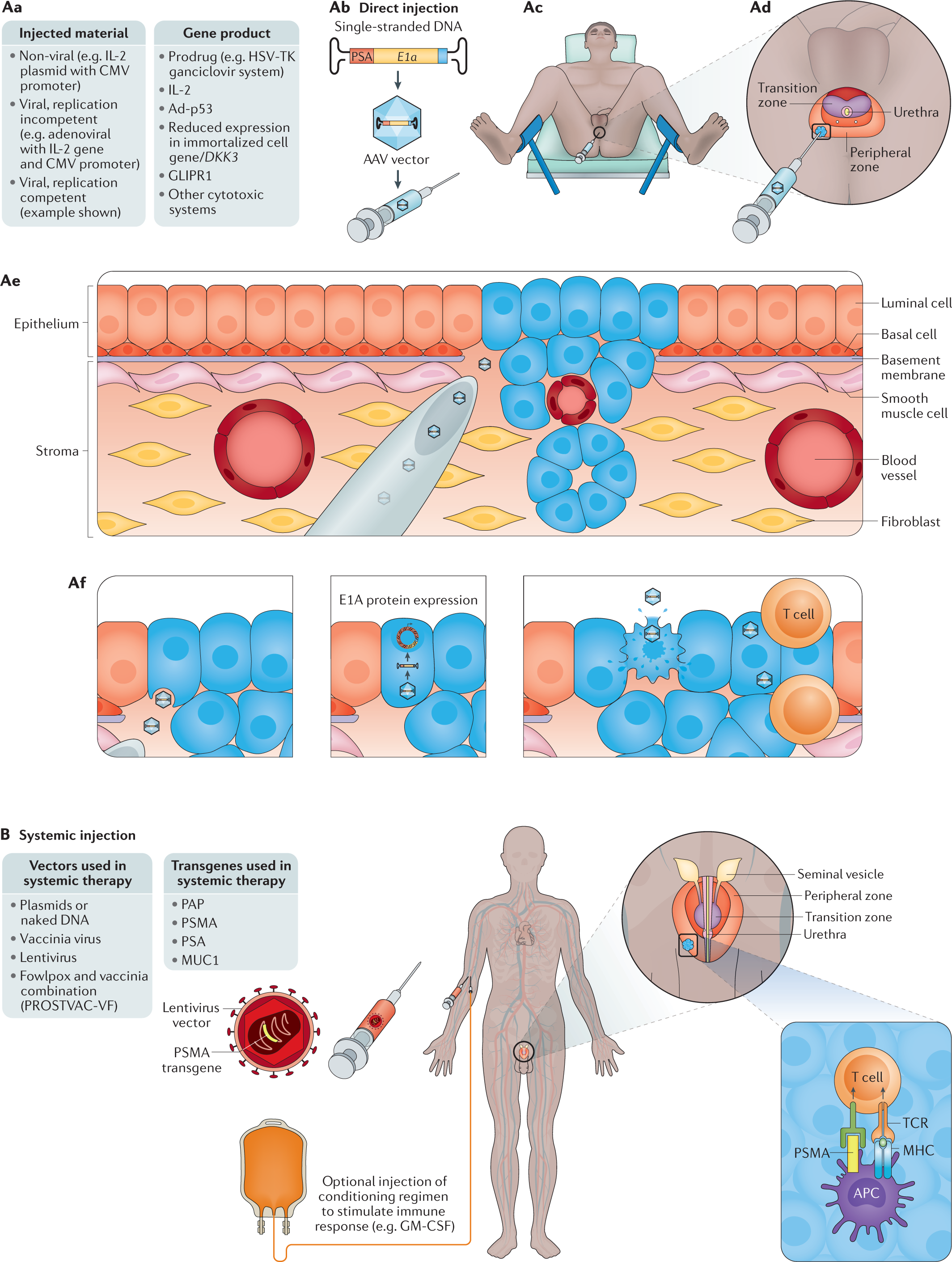
Considering the potential for gene-based therapy in prostate cancer

Detection of cytomegalovirus (CMV) by digital PCR in stool samples for the non-invasive diagnosis of CMV gastroenteritis, Virology Journal

Real-time amplification bioMérieux Clinical Diagnostics
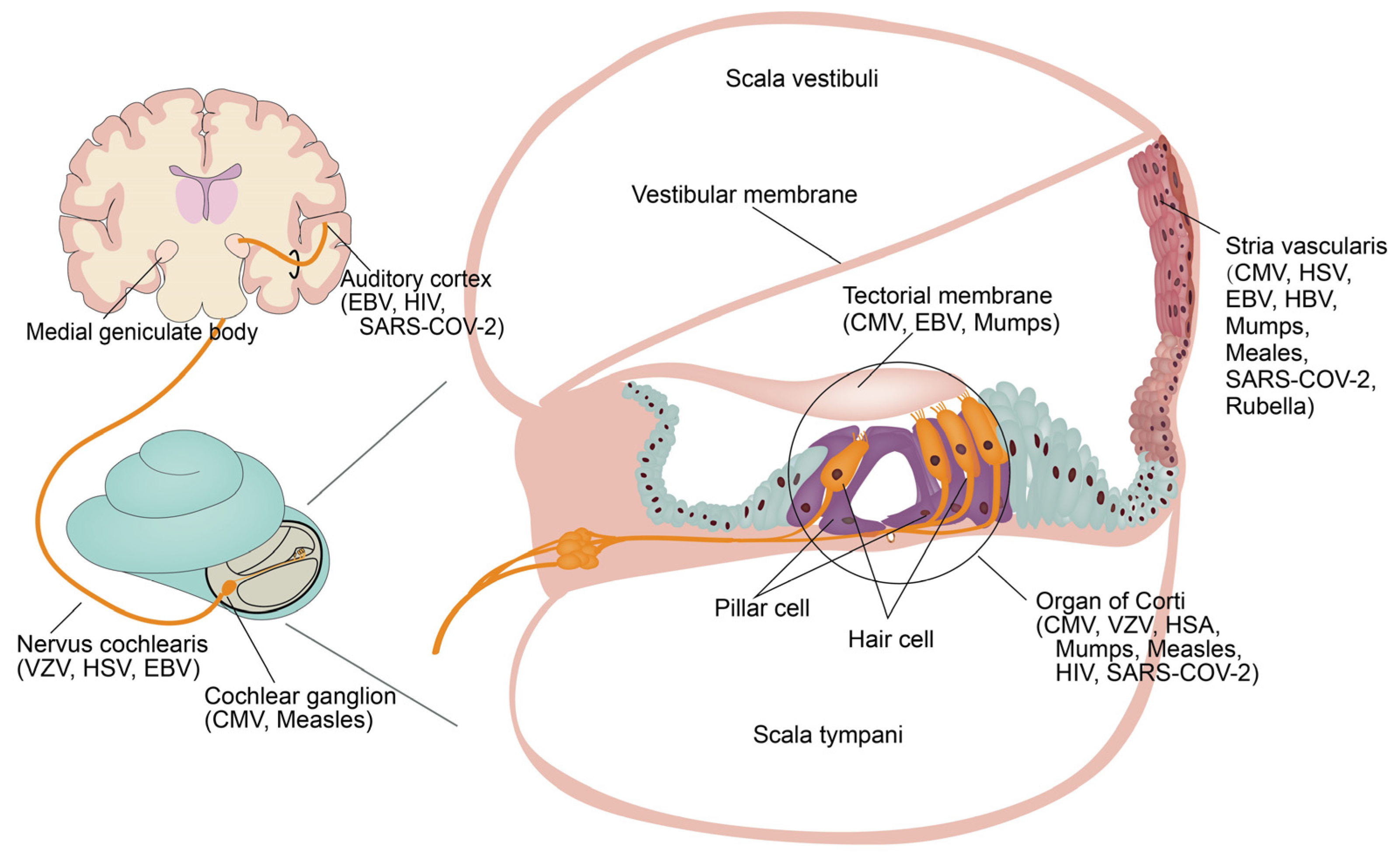
Viruses, Free Full-Text

Parechovirus R-GENE® - clinical diagnostics products

PDF) Virologic Suppression Measured by a CMV DNA Test Calibrated to the WHO International Standard is Predictive of CMV Disease Resolution in Transplant Recipients.

Eliminating HIV-1 Packaging Sequences from Lentiviral Vector Proviruses Enhances Safety and Expedites Gene Transfer for Gene Therapy: Molecular Therapy

CMV R-GENE® - clinical diagnostics products

IJNS, Free Full-Text

Interlaboratory Comparison of Cytomegalovirus Viral Load Assays - American Journal of Transplantation

Frequency counts and correlations between CMV newborn screening

CMV R-GENE® - clinical diagnostics products
Cytomegalovirus Infection - Symptoms, Causes, Treatment

Prevention of Primary Cytomegalovirus Infection in Pregnancy - eBioMedicine

Polymerase chain reaction analysis of aqueous humor specimens in the diagnosis of cytomegalovirus retinitis in AIDS patients - Feifei Mao, Huiyu Sun, Dan Li, Shengnan Wang, Dan Lu, 2020