Coordinate and covalent bond of N2o
4.8 (496) · € 17.99 · En Stock
Coordinate and covalent bond of N2o

N2O Lewis Structure; how can N form a double bond? - CHEMISTRY COMMUNITY

Chemistry-8.Chemical Bonding

Rhenium‐Mediated Conversion of Dinitrogen and Nitric Oxide to Nitrous Oxide - Alig - 2022 - Angewandte Chemie International Edition - Wiley Online Library
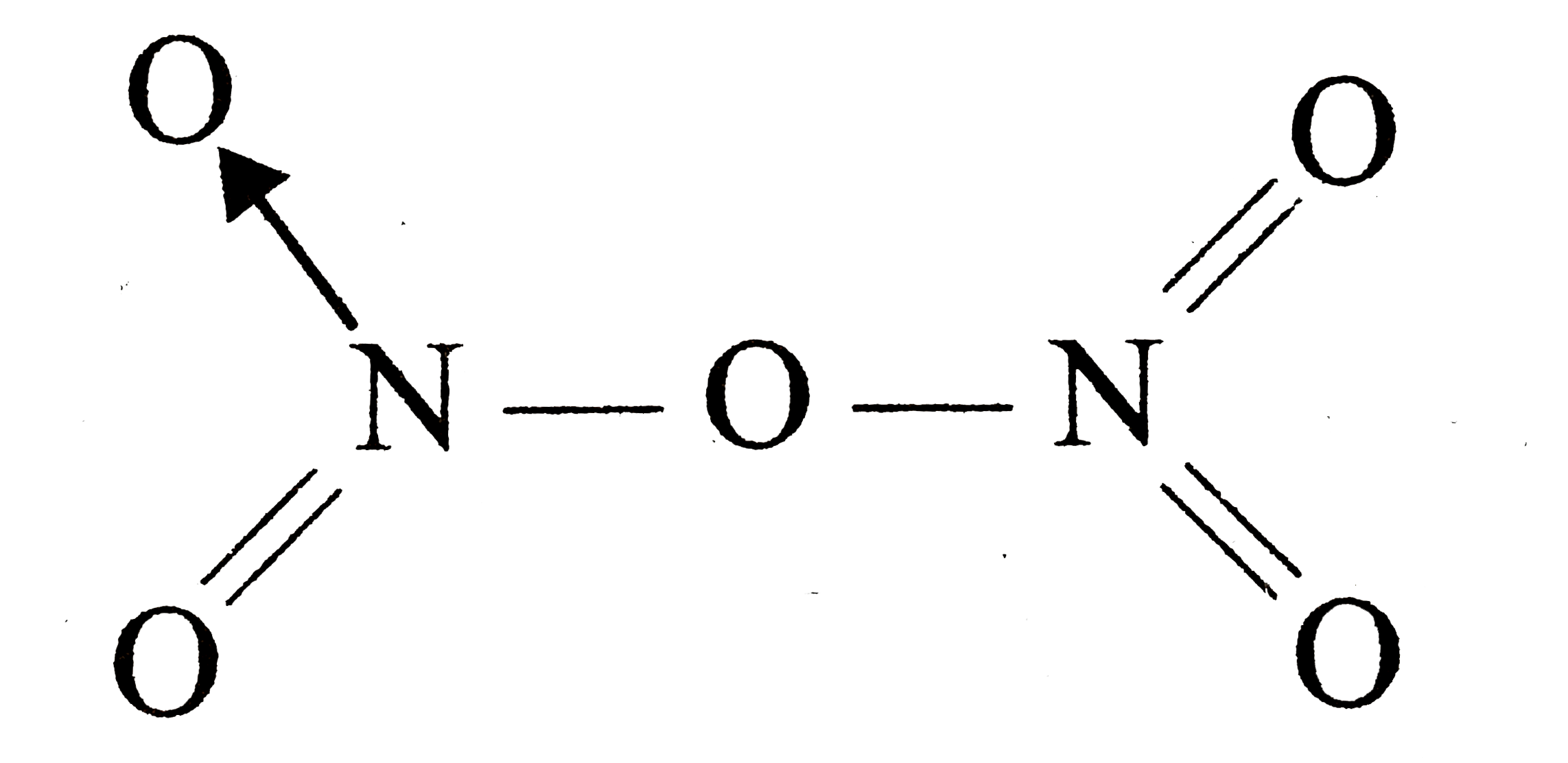
The bonds present in N(2)O(3) (g) are
Is nitrous oxide an ionic compound? Why? - ECHEMI

Draw the Lewis structure { NO }_{ 2 }^{ - }.
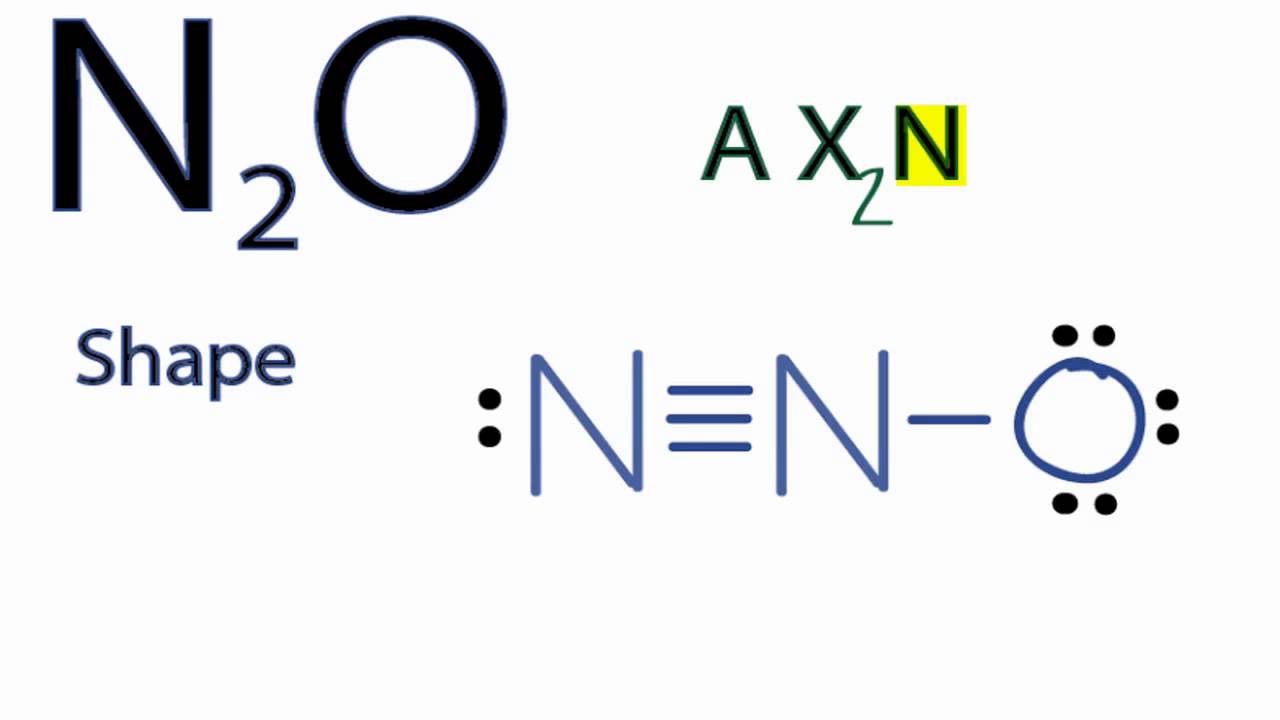
N2O Molecular Geometry / Shape and Bond Angles

Solved Year 11 Chemistry Question 5 (1 marks) Dinitrogen
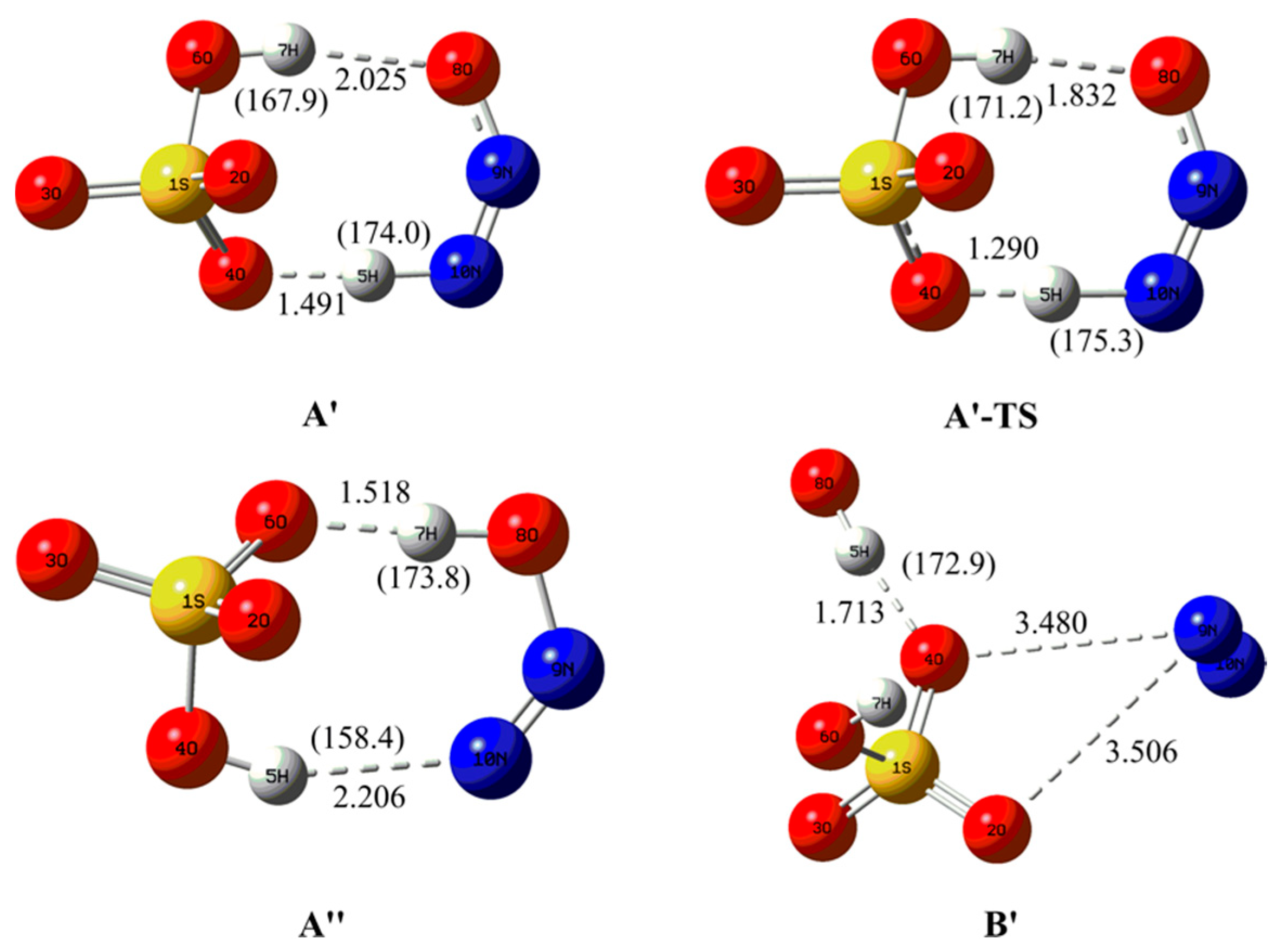
Molecules, Free Full-Text
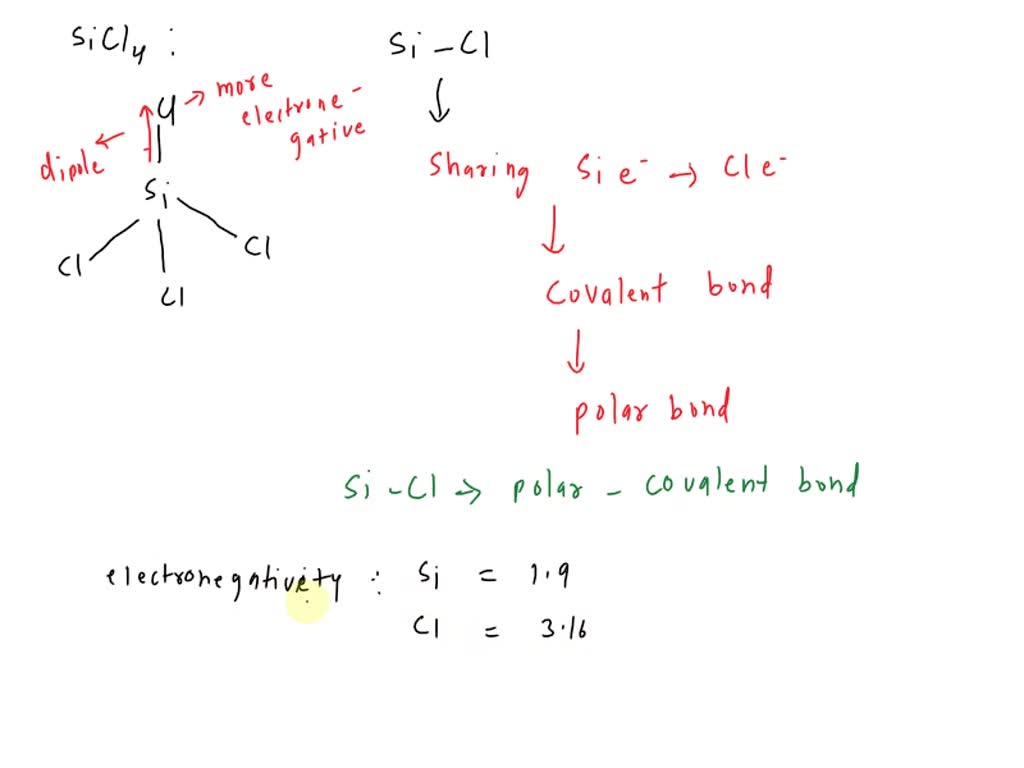
SOLVED: (b) silicon-chlorine bond in SiCl2: nonpolar covalent polar covalent ionic The electronegativity difference for the silicon-chlorine bond is (c) nitrogen-oxygen bond in N2O: nonpolar covalent polar covalent ionic The electronegativity difference

How does the bonding work in N2O? : r/chemhelp