What is Fluorescence? Office for Science and Society - McGill
4.6 (559) · € 37.00 · En Stock
Fluorescence is the ability of certain chemicals to give off visible light after absorbing radiation which is not normally visible, such as ultraviolet light. This property has led to a variety of uses. Let's shed some further light on this topic; consider the omnipresent "fluorescent" lights. Just how do they work? Fluorescent tubes contain a small amount of mercury vapor. The application of an electric current causes a stream of electrons to traverse the tube. These collide with the mercury atoms which become energized and consequently emit ultraviolet light. The inside of the tube is coated with a fluorescent material, such as calcium chlorophosphate, which converts the invisible ultraviolet light into visible light. The same idea is used to produce color television pictures. The screen is coated with tiny dots of substances which fluoresce in different colours when they are excited by a beam of electrons which is used to scan the picture. But fluorescent materials had practical uses even before we dreamed of color television. One of the most amazing of all fluorescent materials is a synthetic compound, appropriately called fluorescein. Under ultraviolet light it produces an intense yellow-green fluorescence which during World War II was responsible for saving the lives of many downed flyers. Over a million pounds of the stuff were manufactured in 1943 and distributed to airmen in little packets to use as a sea marker. Since the fluorescence is so potent that it can be seen when the concentration of fluorescein is as little as 25 parts per billion, rescue planes easily spotted the men in the ocean. Aircraft carriers also made extensive use of fluorescein. The signal men on deck wore clothes and waved flags treated with the compound which was then made to glow by illumination with ultraviolet light. The incoming pilots could clearly see the deck and the need to use runway lights which would have drawn the attention of enemy aircraft was eliminated. Certain natural substances also fluoresce under ultraviolet light. Urine and moose fur are interesting examples. Prisoners have actually made use of this property of urine and have used it as a secret ink. What about the moose fur? Well, in Canada and Sweden there are hundreds of accidents each year involving the collision of automobiles with moose. Some of these result in fatalities. Some car manufacturers are now considering fitting their vehicles with UV emitting headlights to reduce moose collisions! How's that for putting the right chemistry to work.

OSS Events Office for Science and Society - McGill University

Faculty of Medicine & Health Sciences - Graduate Program Discovery Night

Articles by all authors Office for Science and Society - McGill University

Chemical Society Seminar: Francois St-Pierre - Evolved fluorescent indicators to monitor neuronal voltage dynamics in vivo
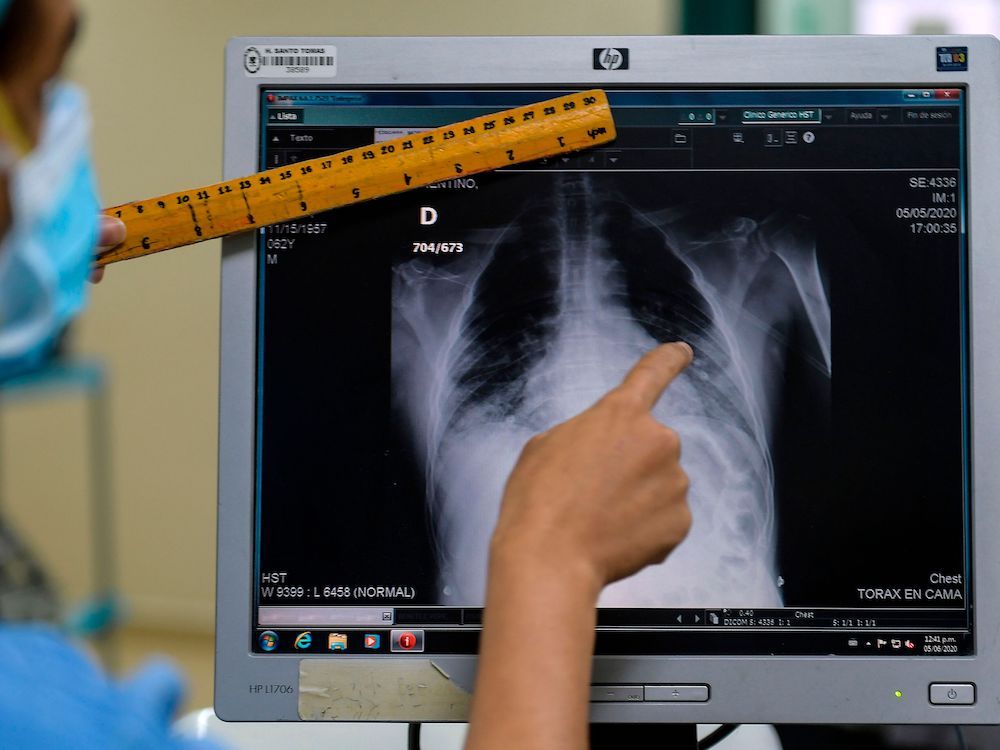
The Right Chemistry: Discovery of X-ray changed medicine forever

Chemical Society Seminar: Eli Zysman-Colman - The design of thermally activated delayed fluorescent and heavy-atom free room temperature phosphorescent supramolecular assemblies
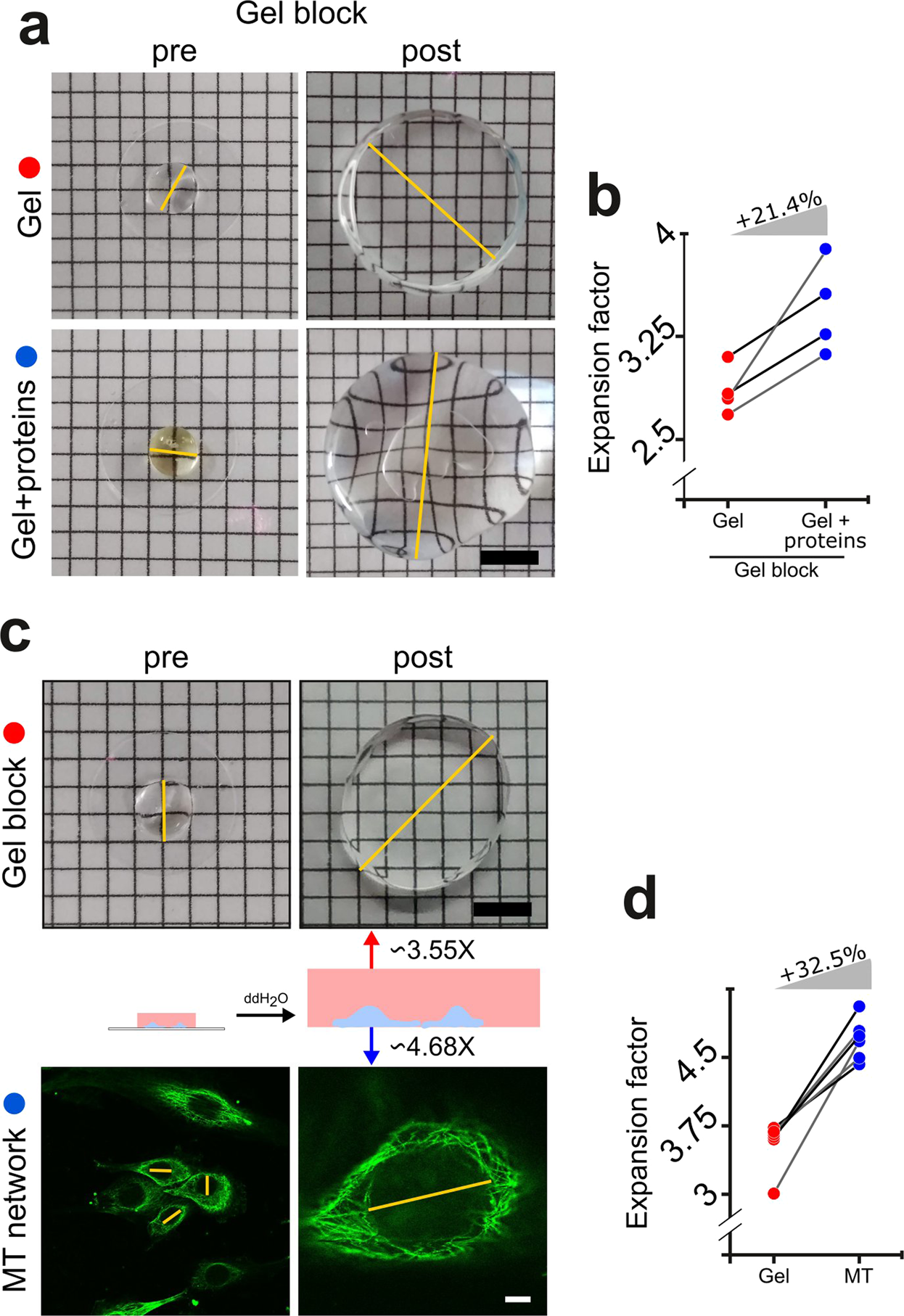
Quantitative expansion microscopy for the characterization of the spectrin periodic skeleton of axons using fluorescence microscopy

MSURJ 2012-2013 by McGill Science Undergraduate Research Journal - Issuu

June 18, 2020) COVID and More: Conversations with the McGill OSS

Single-Particle Resolution Fluorescence Microscopy of Nanoplastics

Rocks in your head? Sort of. Office for Science and Society - McGill University, rocks









:max_bytes(150000):strip_icc()/dan-flavin-weaving-0337f687877d4557a60f1b9324dfa383.jpg)

