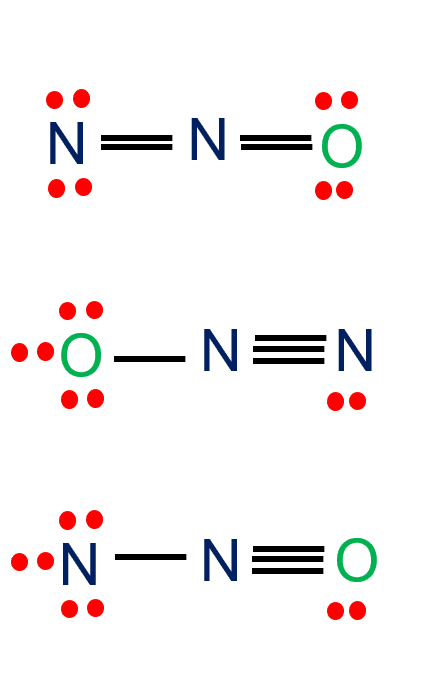
N2 + O2 = N2O - Balanced Chemical Equation
4.7 (796) · € 18.00 · En Stock

How to Balance N2 + O2 = N2O3 (Nitrogen gas + Oxygen gas)

The number of moles of N2O produced when 26.5 g N2 reacts with excess oxygen?

Re-Examination of the N2O + O Reaction
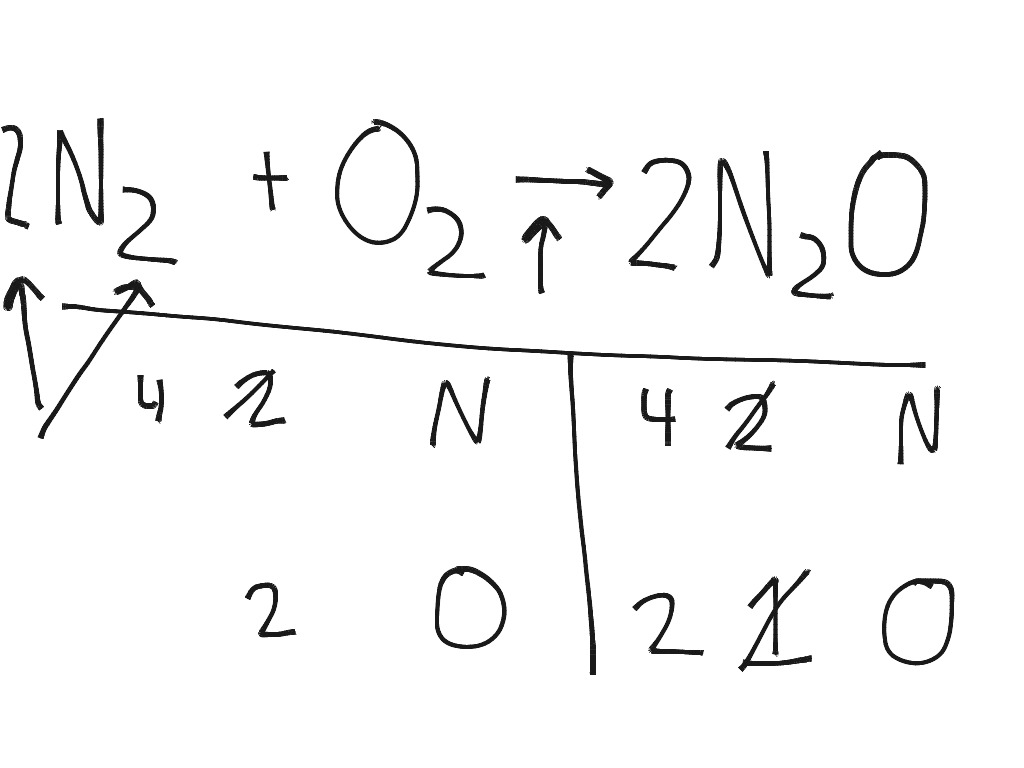
N2 + O2 yield sign N2O chemical equation balancing. By: Jackson Fischer, Science
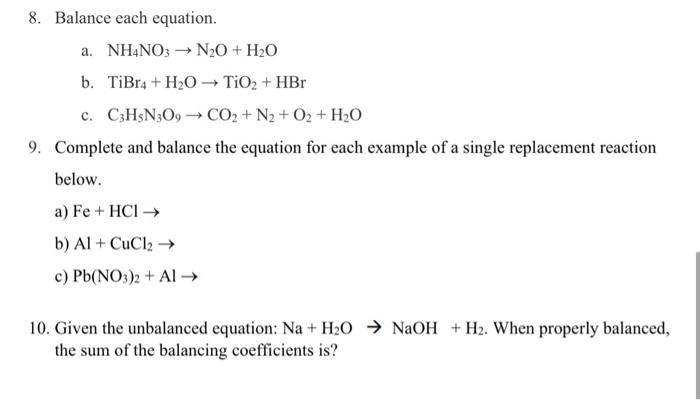
Solved 8. Balance each equation. a. NH4NO3→N2O+H2O b.
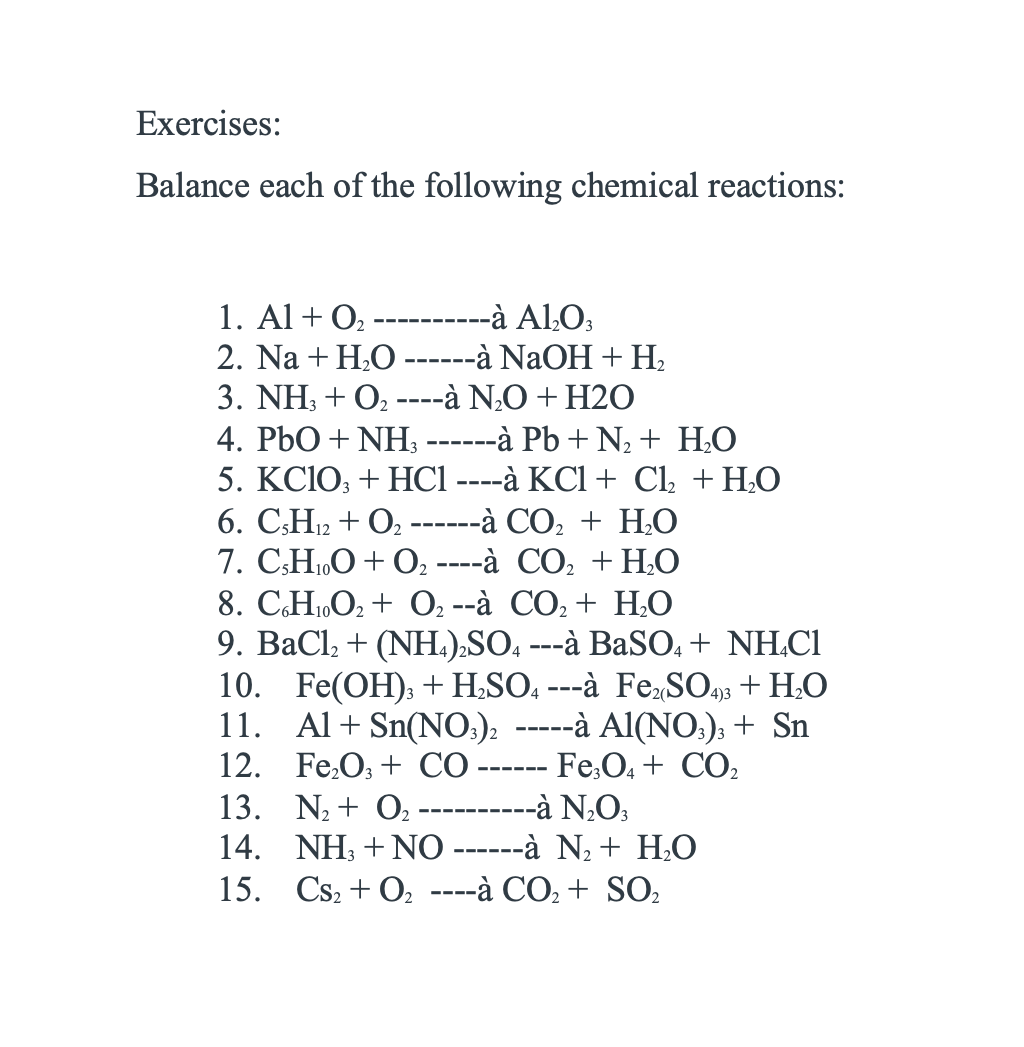
Solved Exercises: Balance each of the following chemical

Rate coefficients of the N2O dissociation leading to N2 plus O products.

How to Balance C3H5(NO3)3 = CO2 + H2O + N2 + O2 (Decomposition of Nitroglycerine)
Reaction between N2 and O2 takes place as follows: 4N2 (g) + O2 (g) 2N2O (g) If a mixture of 0.482 mol N2 and 0.933 mol O2 is placed in a 10L
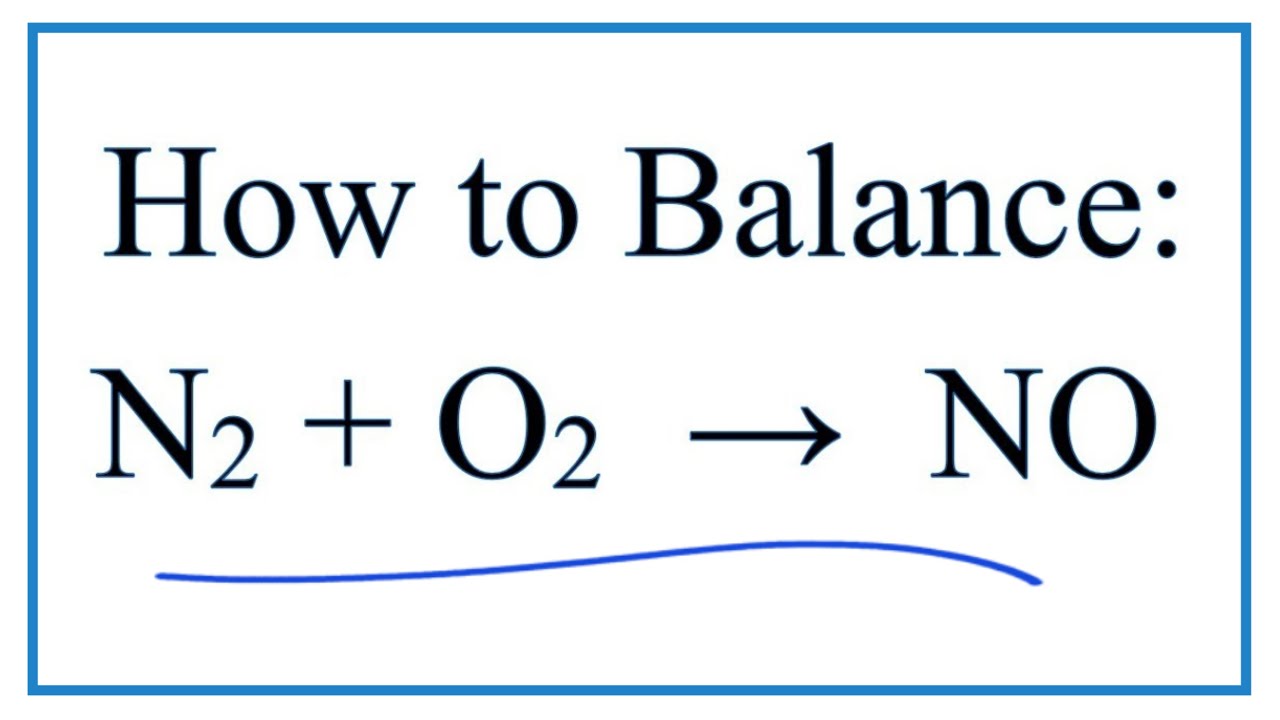
How to Balance O2 + N2 = NO (Oxygen gas + Nitrogen gas)

N2 + O2 = N2O - Balanced Chemical Equation
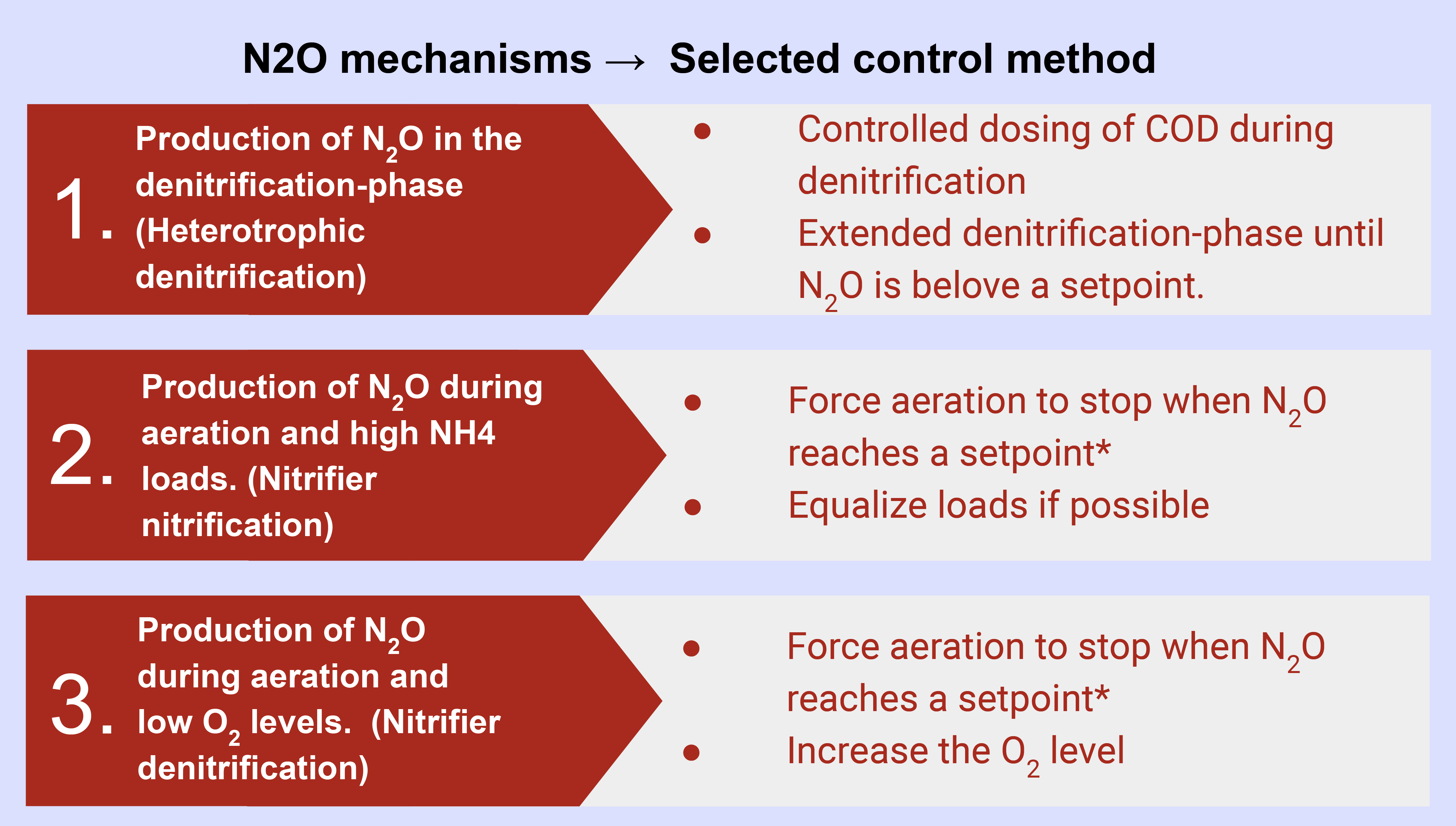
Processes, Free Full-Text

Week 12 Test Review Chemistry. - ppt download
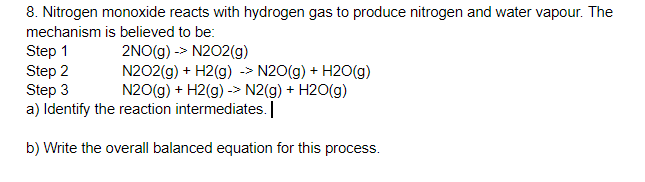
Solved 8. Nitrogen monoxide reacts with hydrogen gas to

SOLVED: When N2(g) reacts with O2(g) to form N2O(g), 19.6 kcal of energy are absorbed for each mole of N2(g) that reacts. Write a balanced equation for the reaction with an energy