Update on REMS-Required Testing During COVID-19 Pandemic - MPR
4.8 (606) · € 21.99 · En Stock
“The completion of some REMS-required laboratory testing or imaging studies may be difficult because patients suspected of having COVID-19 may be self-isolating and/or subject to quarantine,” said FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD.

Archived Webinars Readiness and Emergency Management for Schools Technical Assistance Center
After the Public Health Emergency: FDA plans to revise COVID-19 EUA policies - Hogan Lovells Engage

The utility of the Rapid Emergency Medicine Score (REMS) compared with three other early warning scores in predicting in-hospital mortality among COVID-19 patients in the emergency department: a multicenter validation study

Archived Webinars Readiness and Emergency Management for Schools Technical Assistance Center

Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine
Clozapine During COVID-19: How Best to Ensure Patient Safety

URAC COVID-19 Updates - What You Need To Know
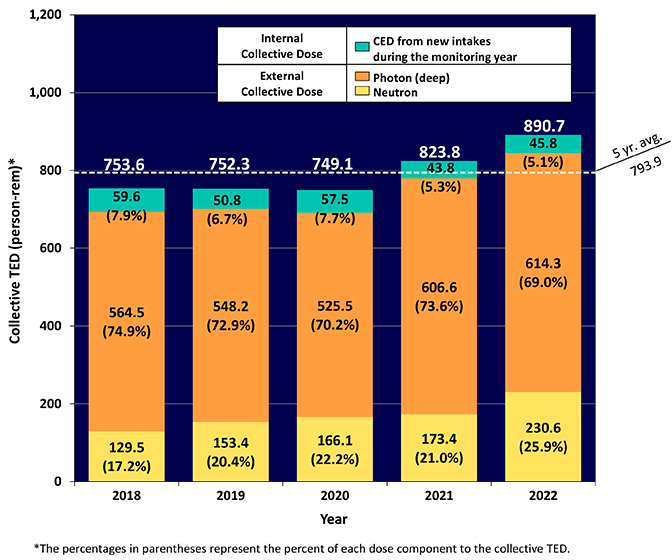
2021 Occupational Radiation Exposure Dashboard

Falsification of at-home isotretinoin pregnancy testing during the COVID-19 pandemic: A case series and proposal of mitigation strategies - ScienceDirect

Risk Evaluation and Mitigation Strategy programs: How they can be improved

REMS Changes Due to the COVID-19 Pandemic

COVID-19 Resource Center - Texas Hospital Association

The Risk Evaluation and Mitigation Strategy (REMS) Public Dashboard: Improving Transparency of Regulatory Activities