Understanding The FDA's Current Focus On Risk Evaluation And
4.8 (531) · € 36.50 · En Stock
lt;p>The FDA recently asked for comments about how the government handles vendor change requests from drug sponsors with risk evaluation and mitigation strategies. So, we asked a REMS expert to help us understand why the agency is focusing on the broad-reaching program and what it could mean for drug manufacturers with REMS products in their portfolios.</p>

FDA's Recent Benefit Risk Assessment Guidance Explained

Food and Drug Administration - Wikipedia

Aggregate Reports: USFDA structured Approach to Benefit-Risk

FDA Evaluations of Medical AI Devices Show Limitations

US FDA approvals bounce back in 2023, sparking hopes of a biotech
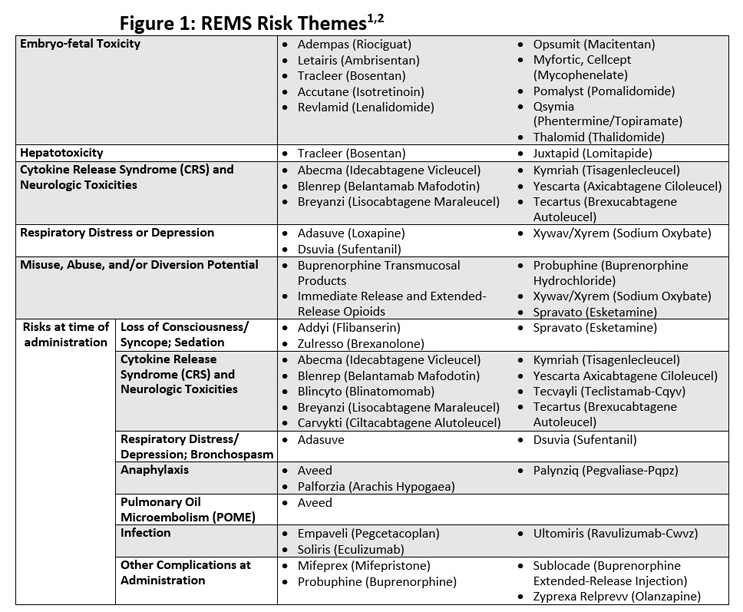
Avoid Launch Delays By Planning For An FDA-Required REMS Risk
Frontiers New science, drug regulation, and emergent public

Understanding The FDA's Current Focus On Risk Evaluation And

FDA's own documents reveal agency's lax, slow, and secretive

Summary of Guidance for Minimizing the Impact of COVID-19 on

Understanding the Differences between Hazard Analysis and Risk










