- Accueil
- mcv4
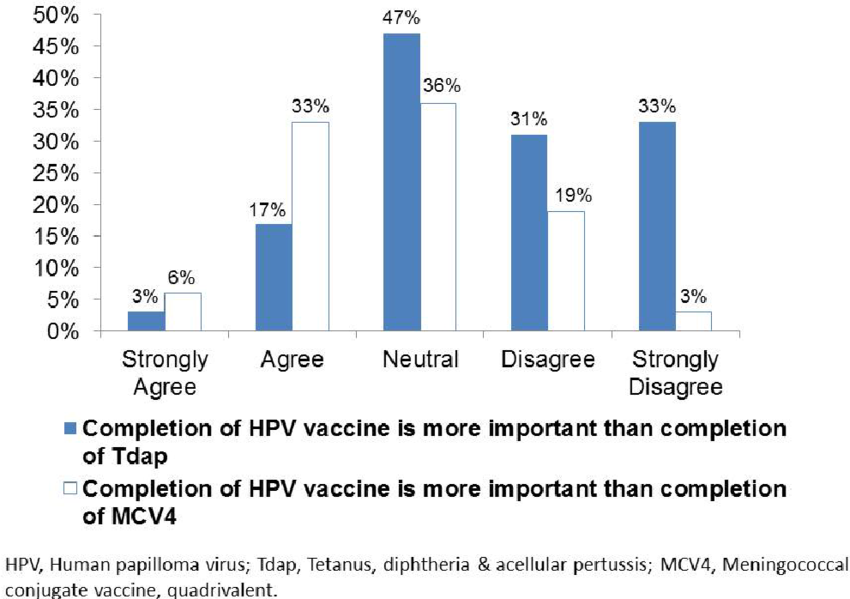
- Immunogenicity and reactogenicity of co-administered tetanus–diphtheria–acellular pertussis (Tdap) and tetravalent meningococcal conjugate (MCV4) vaccines compared to their separate administration - ScienceDirect
Immunogenicity and reactogenicity of co-administered tetanus–diphtheria–acellular pertussis (Tdap) and tetravalent meningococcal conjugate (MCV4) vaccines compared to their separate administration - ScienceDirect
4.5 (404) · € 30.50 · En Stock

Full article: Immunogenicity, safety, and reactogenicity of combined reduced-antigen-content diphtheria-tetanus-acellular pertussis vaccine administered as a booster vaccine dose in healthy Russian participants: a phase III, open-label study
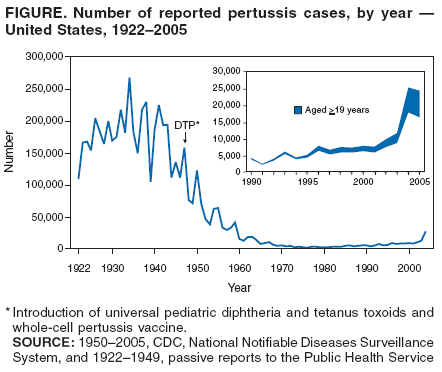
Preventing Tetanus, Diphtheria, and Pertussis Among Adults: Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine
Recommendations of the Advisory Committee on Immunization Practices (ACIP) and Recommendation of ACIP

Vaccination - ScienceDirect
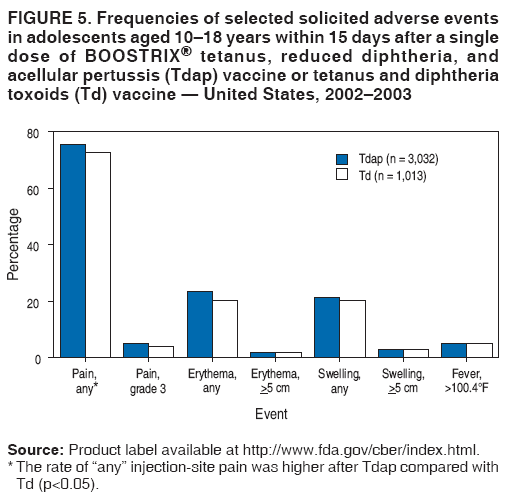
Prevention of Pertussis, Tetanus, and Diphtheria Among Pregnant and Postpartum Women and their Infants Recommendations of the Advisory Committee on Immunization Practices (ACIP)

PDF) The adjuvanted recombinant zoster vaccine co-administered with a tetanus, diphtheria and pertussis vaccine in adults aged ≥50 years: A randomized trial
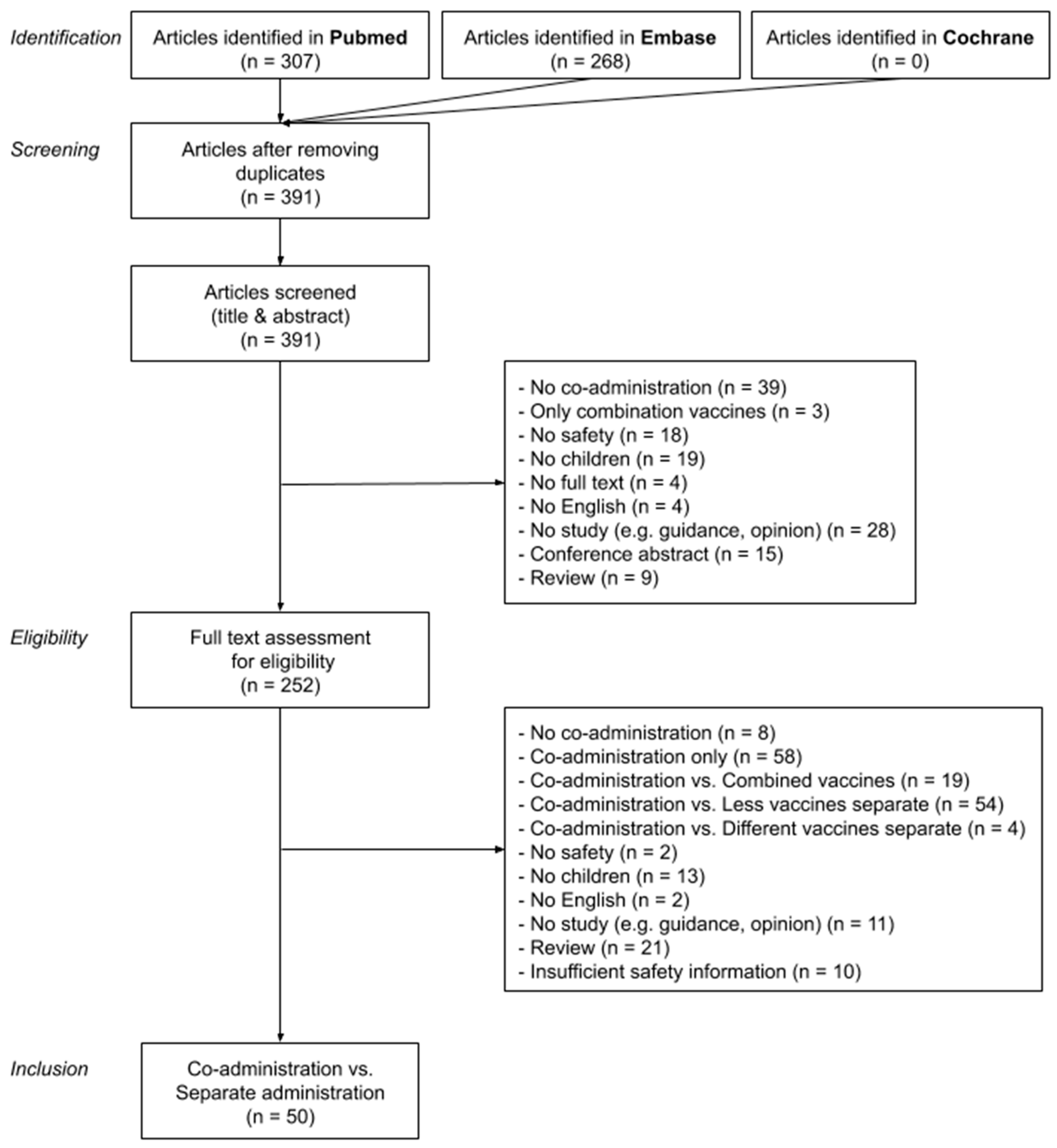
Vaccines, Free Full-Text
Vaccines, Free Full-Text
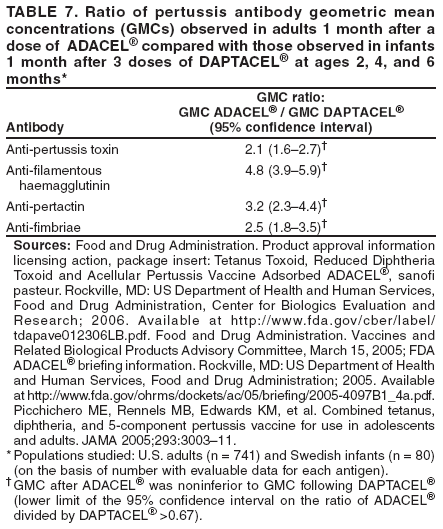
Preventing Tetanus, Diphtheria, and Pertussis Among Adults: Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine
Recommendations of the Advisory Committee on Immunization Practices (ACIP) and Recommendation of ACIP
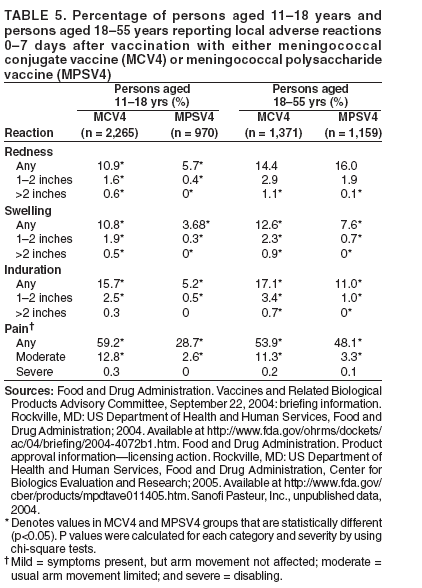
Prevention and Control of Meningococcal Disease
Recommendations of the Advisory Committee on Immunization Practices (ACIP)