- Accueil
- mcv4
- PDF) Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age
PDF) Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age
4.7 (692) · € 30.50 · En Stock
PDF | Routine administration of quadrivalent meningococcal conjugate vaccine to adolescents and certain high risk groups is recommended in the United | Find, read and cite all the research you need on ResearchGate

PDF) Safety and Immunogenicity of Quadrivalent Meningococcal Conjugate Vaccine in 2- to 10-year-old Human Immunodeficiency Virus-infected Children

Immune response, antibody persistence, and safety of a single dose of the quadrivalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine in adolescents and adults: results of an open

Emerging Topics in Vaccine Therapeutics for Adolescents and Adults: An Update for Immunizing Pharmacists - Joseph P. Fava, Brittany Stewart, Kathryn M. Dudzinski, Michelle Baker, Lucio Volino, 2020

Full article: Immunogenicity and safety of an investigational quadrivalent meningococcal conjugate vaccine administered as a booster dose in children vaccinated against meningococcal disease 3 years earlier as toddlers: A Phase III, open-label

An evaluation of emerging vaccines for childhood meningococcal disease, BMC Public Health

Microorganisms, Free Full-Text
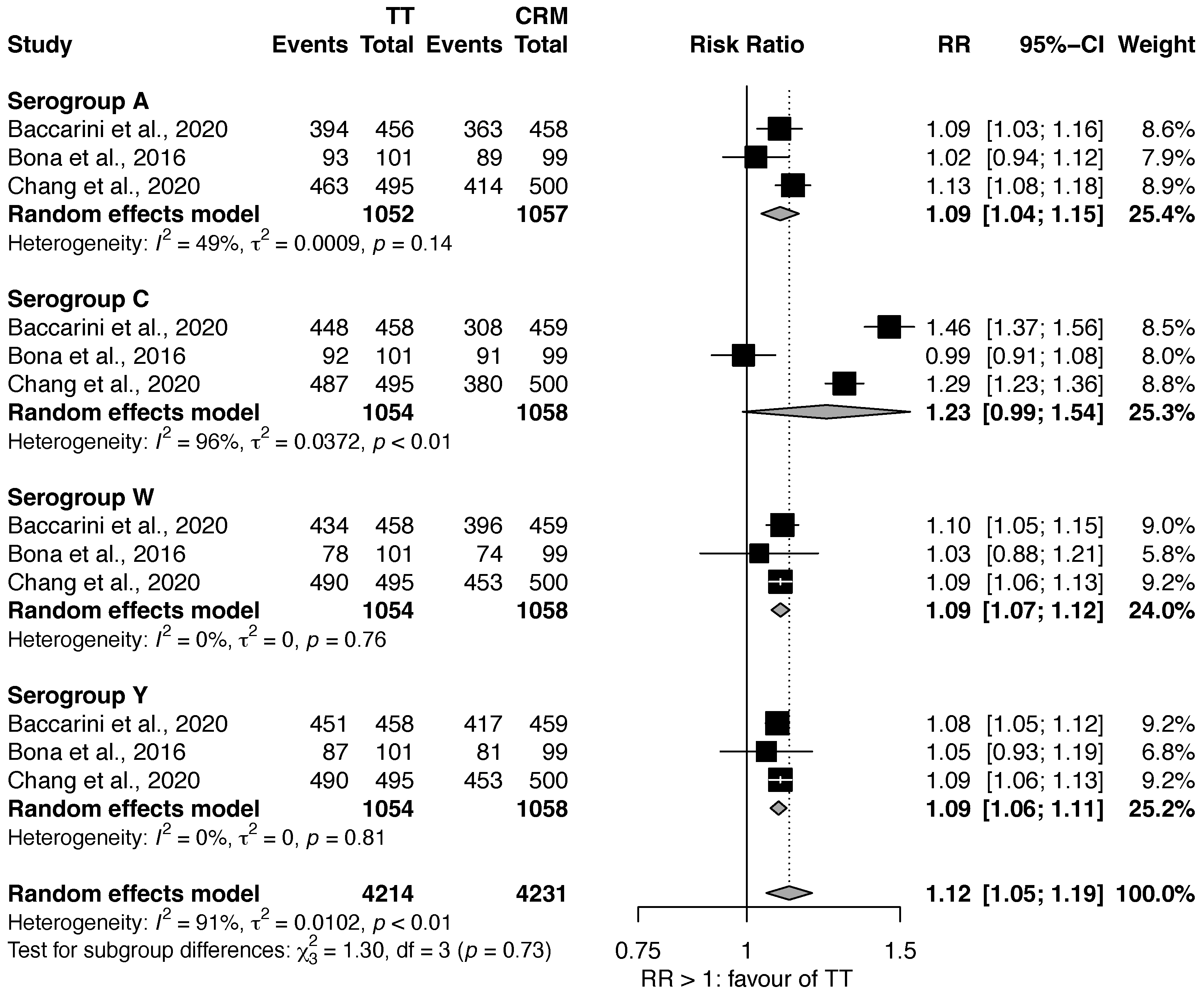
Vaccines, Free Full-Text

Full article: Immunogenicity and safety of an investigational quadrivalent meningococcal conjugate vaccine administered as a booster dose in children vaccinated against meningococcal disease 3 years earlier as toddlers: A Phase III, open-label

Vaccines, Free Full-Text

Full article: Immunogenicity and safety of an investigational quadrivalent meningococcal conjugate vaccine administered as a booster dose in children vaccinated against meningococcal disease 3 years earlier as toddlers: A Phase III, open-label












