- Accueil
- mcv4
- Full article: Comparing the meningococcal serogroup C immune response elicited by a tetanus toxoid conjugate quadrivalent meningococcal vaccine (MenACYW-TT) versus a quadrivalent or monovalent C tetanus toxoid conjugate meningococcal vaccine in healthy
Full article: Comparing the meningococcal serogroup C immune response elicited by a tetanus toxoid conjugate quadrivalent meningococcal vaccine (MenACYW-TT) versus a quadrivalent or monovalent C tetanus toxoid conjugate meningococcal vaccine in healthy
4.6 (109) · € 25.50 · En Stock

Meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate v

Full article: Immunogenicity and safety of an investigational quadrivalent meningococcal conjugate vaccine administered as a booster dose in children vaccinated against meningococcal disease 3 years earlier as toddlers: A Phase III, open-label
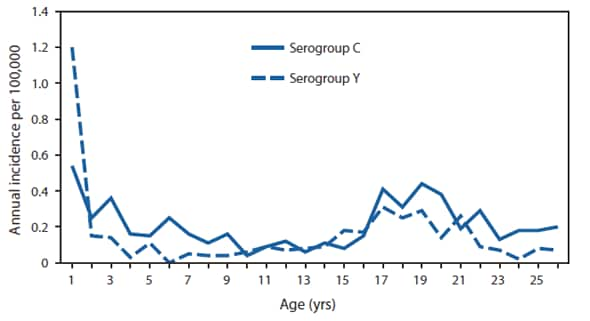
Updated Recommendations for Use of Meningococcal Conjugate Vaccines --- Advisory Committee on Immunization Practices (ACIP), 2010

Factors contributing to the immunogenicity of meningococcal conjugate vaccines. - Abstract - Europe PMC

Meningococcal ACWYX Conjugate Vaccine in 2-to-29-Year-Olds in Mali and Gambia
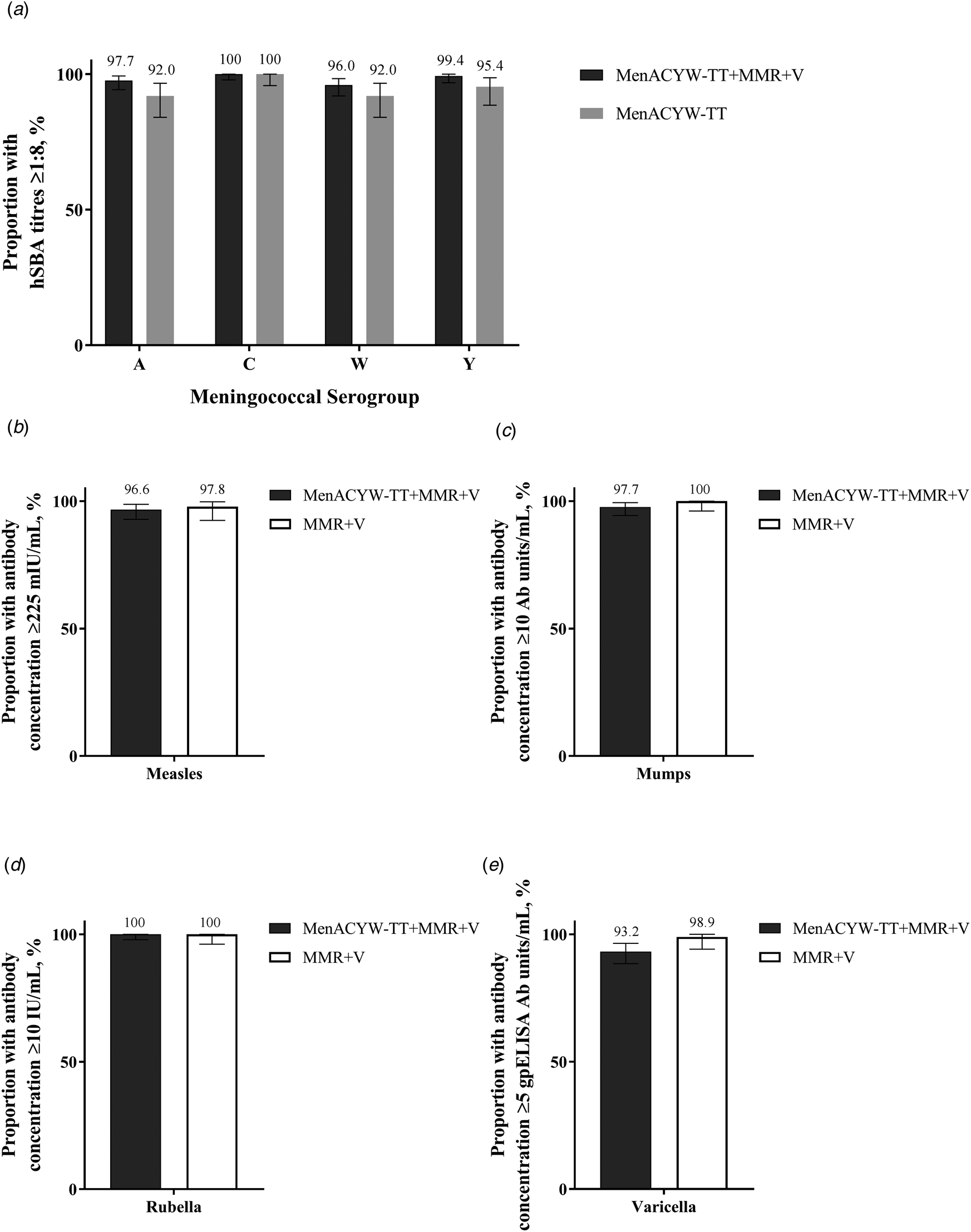
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine administered concomitantly with other paediatric vaccines in toddlers: a phase III randomised study, Epidemiology & Infection

Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in ≥56-year-olds: A Phase III randomized study - ScienceDirect
Meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine: a new conjugate vaccine against invasive meningococcal disease - Document - Gale Academic OneFile

Safety and immunogenicity of a pentavalent meningococcal conjugate vaccine containing serogroups A, C, Y, W, and X in healthy adults: a phase 1, single-centre, double-blind, randomised, controlled study - The Lancet Infectious

Combined effects of glycan chain length and linkage type on the immunogenicity of glycoconjugate vaccines

Preclinical development of the quadrivalent meningococcal (ACYW) tetanus toxoid conjugate vaccine, MenQuadfi®








/https://static.texastribune.org/media/images/2013/08/08/Vaccine-Lead.jpg)
