- Accueil
- g heat
- Ex.18 In the system, LaCl3(s) + H2O(g) + Heat = LaCIO(s) + 2HCl(g), is established. More water vapour is added to reestablish the equilibrium. The pressure of water vapour is doubled. The
Ex.18 In the system, LaCl3(s) + H2O(g) + Heat = LaCIO(s) + 2HCl(g), is established. More water vapour is added to reestablish the equilibrium. The pressure of water vapour is doubled. The
4.9 (395) · € 14.50 · En Stock
Click here:point_up_2:to get an answer to your question :writing_hand:ex18in the systemlacl3s h2og heat lacios 2hclgis established more water vapour is
Click here👆to get an answer to your question ✍️ Ex-18 In the system- LaCl3-s- - H2O-g- - Heat - LaCIO-s- - 2HCl-g- is established- More water vapour is added to reestablish the equilibrium- The pressure of water vapour is doubled- The factor by which pressure of HCl is changed is - -A-2 -B- V -C- 13 -D
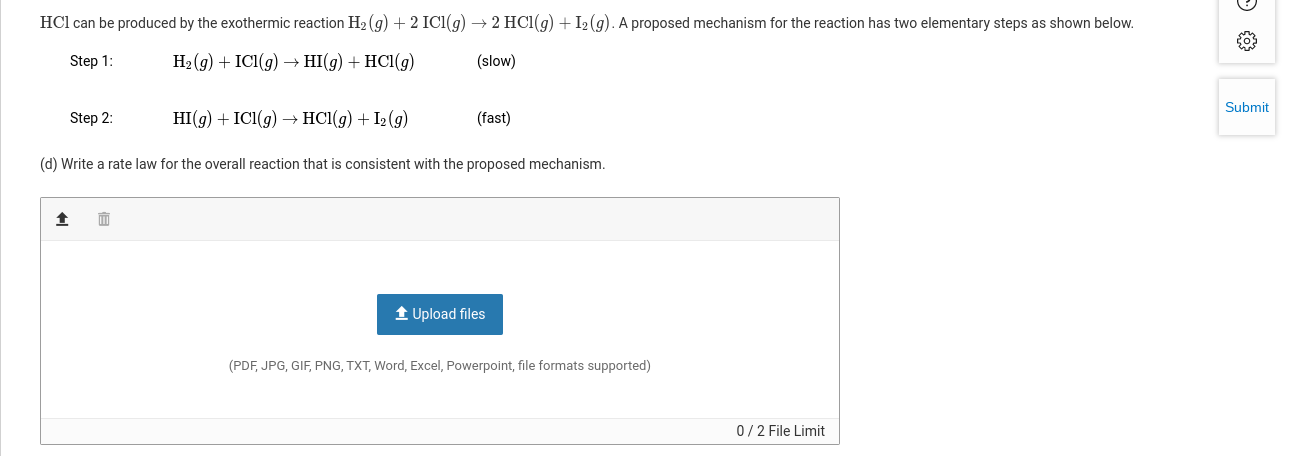
Solved Question 2 For parts of the free-response question
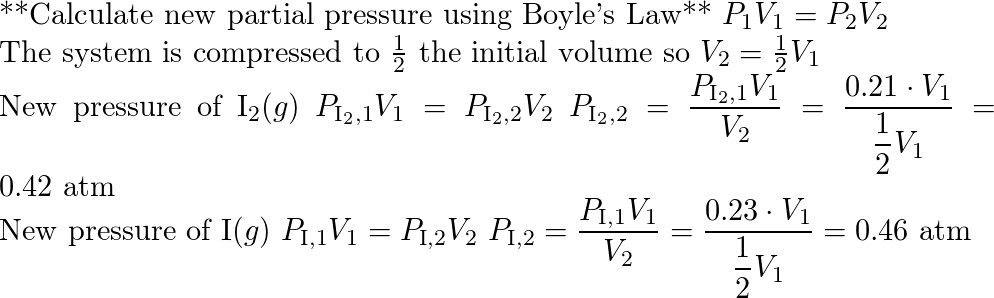
A system at equilibrium contain s $l_2(g)$ at a pressure of

A system at equilibrium contain s $l_2(g)$ at a pressure of

Chemistry 2 Flashcards

16. In the following equilibrium in a closed container: LaClz (s) + H2O(g)- >LaCIO(s) + 2HCl(g) The vapour pressure of H2O is 24 mm 25°C. If pressure of H20 (g) is increased
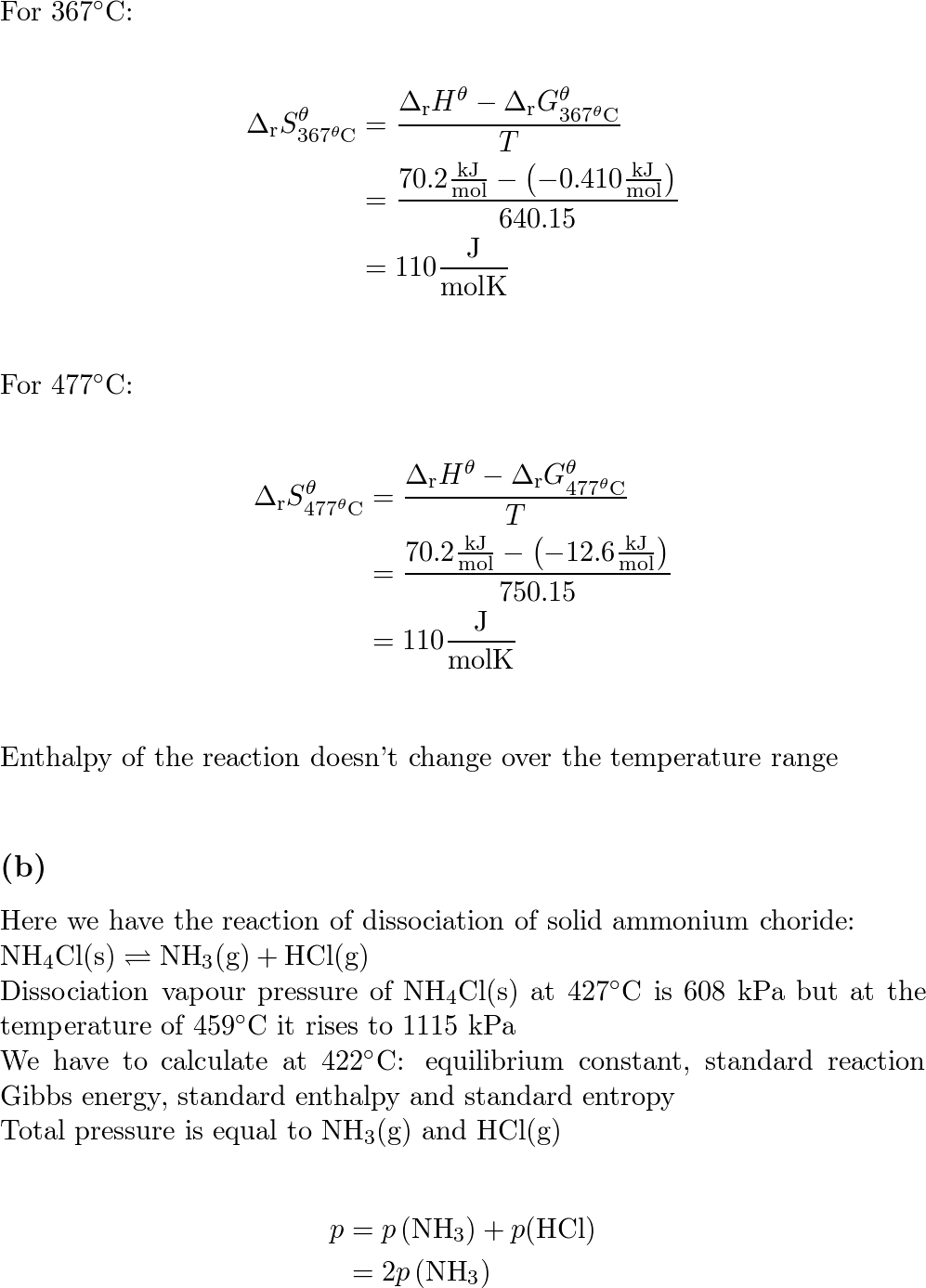

CHAPTER 18 Entropy, Free Energy, and Equilibrium. - ppt download

Chapter 17 Additional Aspects of Aqueous Equilibria - Chapter19 Homework Part A multiple choice - Studocu

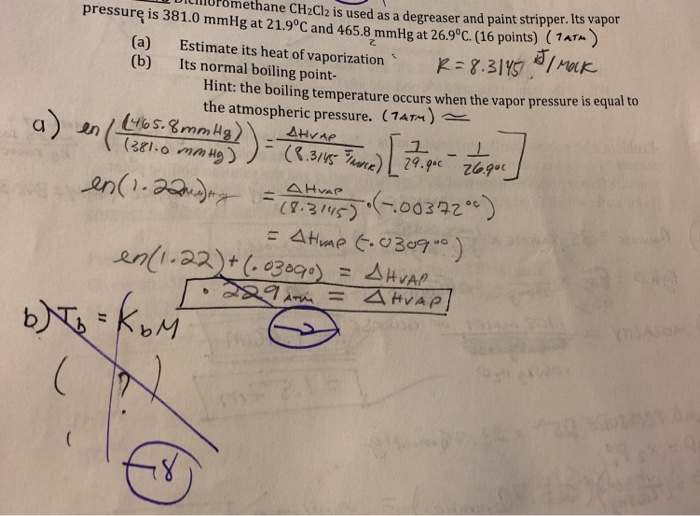
Solved 1. Calculate the heat release by cooling 55.0g H2O

Consider the water gas equilibrium reaction,Cleft( s right) +{ H }_{ 2 }Oleft( g right) rightleftharpoons COleft( g right) +{ H }_{ 2 }left( g right) Which of the following statements is

If 42.0 kJ of heat is added to a 32.0-g sample of liquid meth-ane












