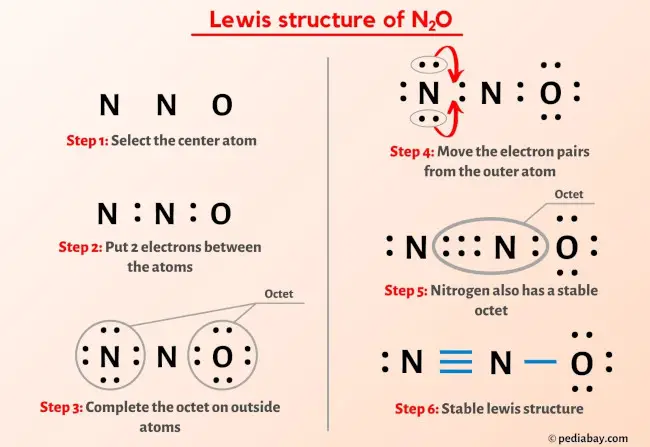
N2O lewis structure, molecular geometry, bond angle, hybridization
4.6 (581) · € 39.50 · En Stock
Nitrous oxide (N2O) Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, hybridization, formal charges, polar vs non-polar

Prediction of Shapes and Bond Angles Chapter 5, PDF, Physical Chemistry

Which of the following molecules have the same geometries? a. CO2 and BeH2 b. SF4 and CH4 c. CO2 and NO2 d. N2O and NO2
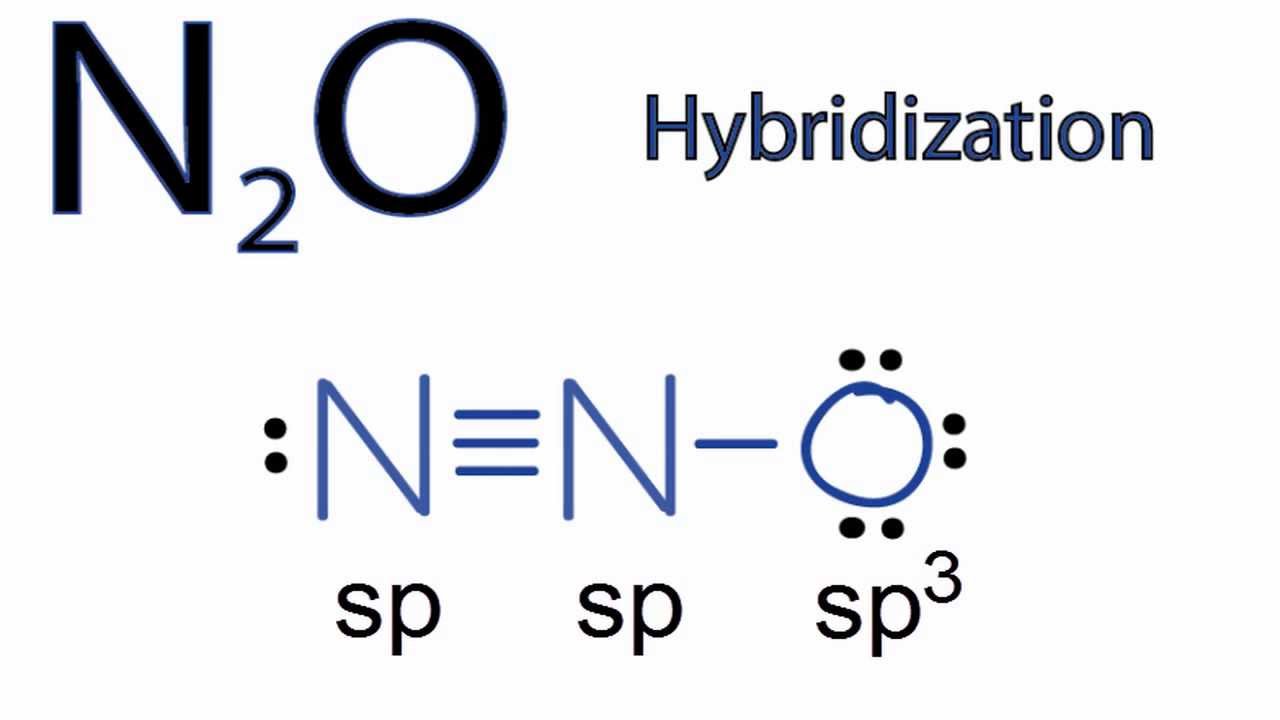
N2O Hybridization: Hybrid Orbitals for N2O

N2O lewis structure, molecular geometry, bond angle, hybridization

N2O4 Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity - Techiescientist

Determine the molecular geometry of N2O(oxygen is terminal). a) tetrahedral b) trigonal planar c) trigonal pyramidal d) bent e) linear

Ch05 Molecular Structure and Orbitals.pdf - finedrafts
What is the hybridisation of N2O? - Quora
What is the hybridisation of N2O? - Quora
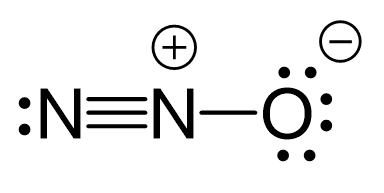
N2O Geometry and Hybridization - Chemistry Steps

Hybridization of NO2 - Hybridization of N in Nitrogen Dioxide

Which two of the following molecules have the same molecular geometry? Please show your work (Lewis structures and 3D molecular shapes) and explain your reasoning. a. N2O b. Se2O c. CO2

N2O Lewis, Shape, Hybridization, Polarity, and more.

Resonance Structures Video Tutorial & Practice

Comparison of the six possible N2O-bound models The models are
/product/49/414146/1.jpg?7505)