Fabrication de produits de diagnostic in vitro
4.9 (530) · € 26.00 · En Stock

Pompes de diagnostic (IVD) In Vitro

LISTES - 1 Liste pdf ach GB - PCH Meetings
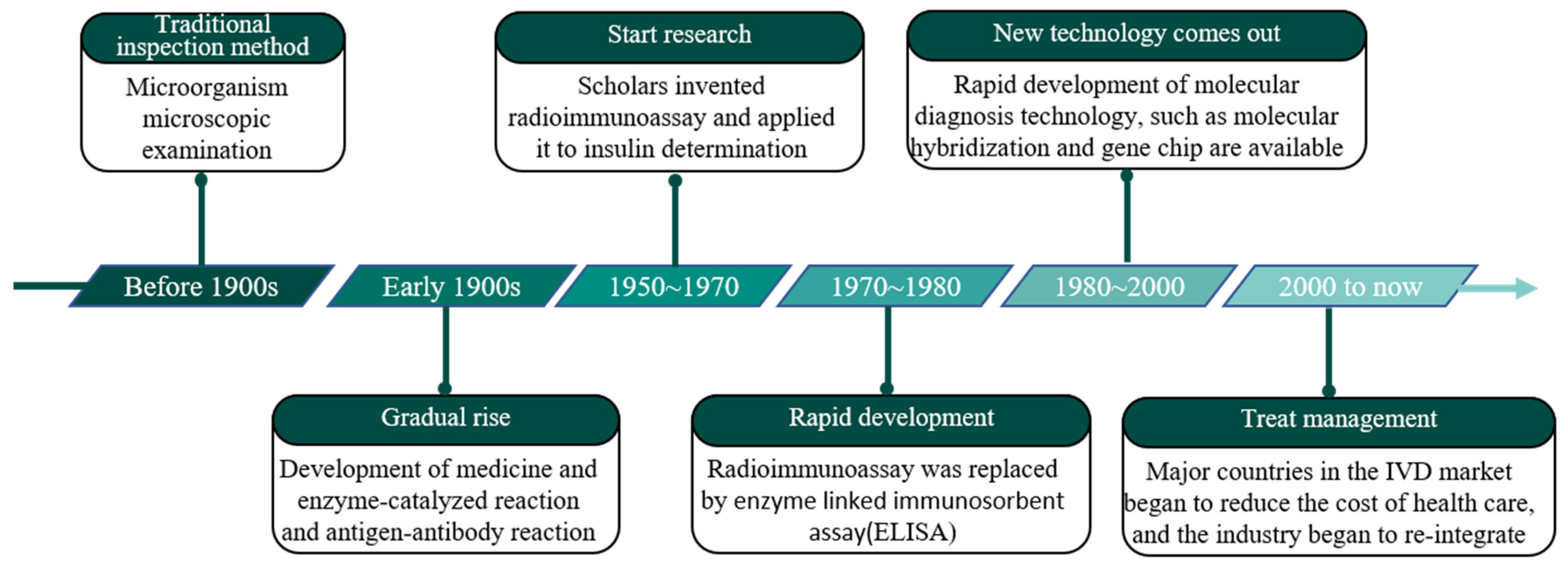
Processes, Free Full-Text

Les dispositifs médicaux de diagnostic in vitro (DMDIV) selon le

Structure of the copolymers used for surface treatment of thiol
ISO 18113-2:2022 - This document specifies requirements for information supplied by the manufacturer of in vitro diagnostic (IVD) reagents,

ISO 18113-2:2022 - In vitro diagnostic medical devices — Information supplied by the manufacturer (labelling) — Part 2: In vitro diagnostic reagents f

SEQENS In Vitro Diagnostic

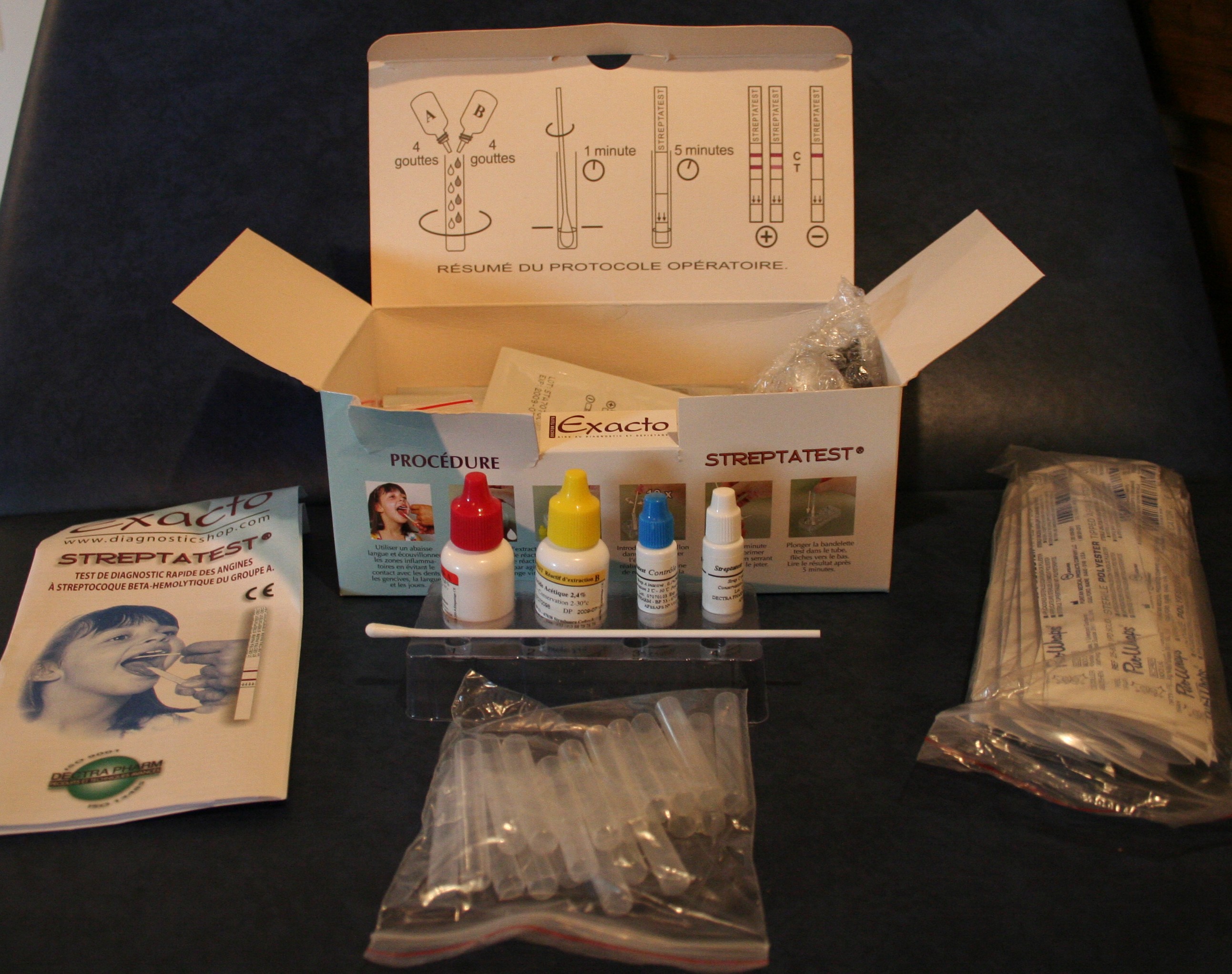
Réactif de diagnostic in vitro (DIV réactifs) Kit de test de

Catalogue-life-2023-2024
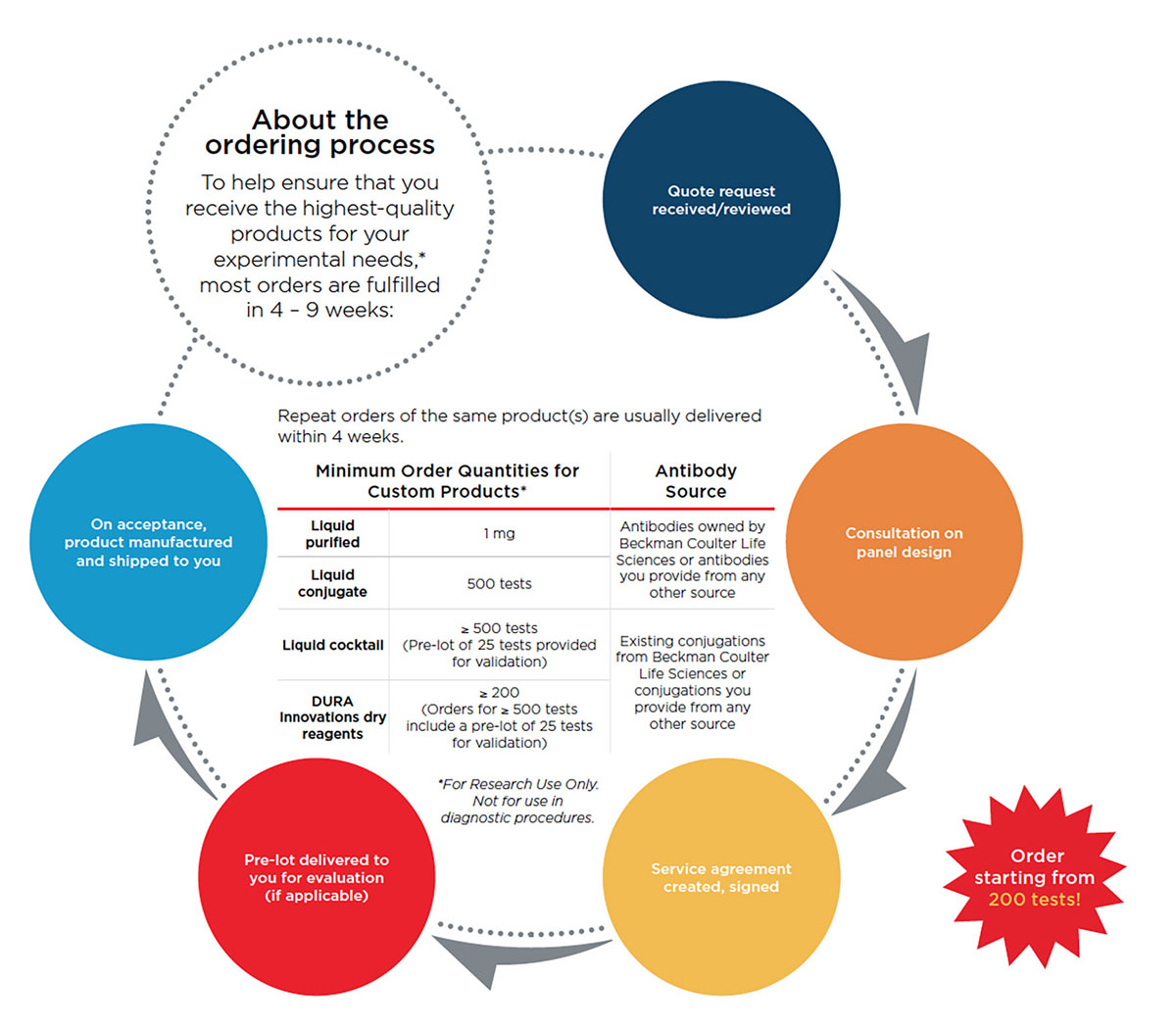
Contract Manufacturing Services - Bulk Orders Made-To

Manufacture, Gene Therapy Manufacturing

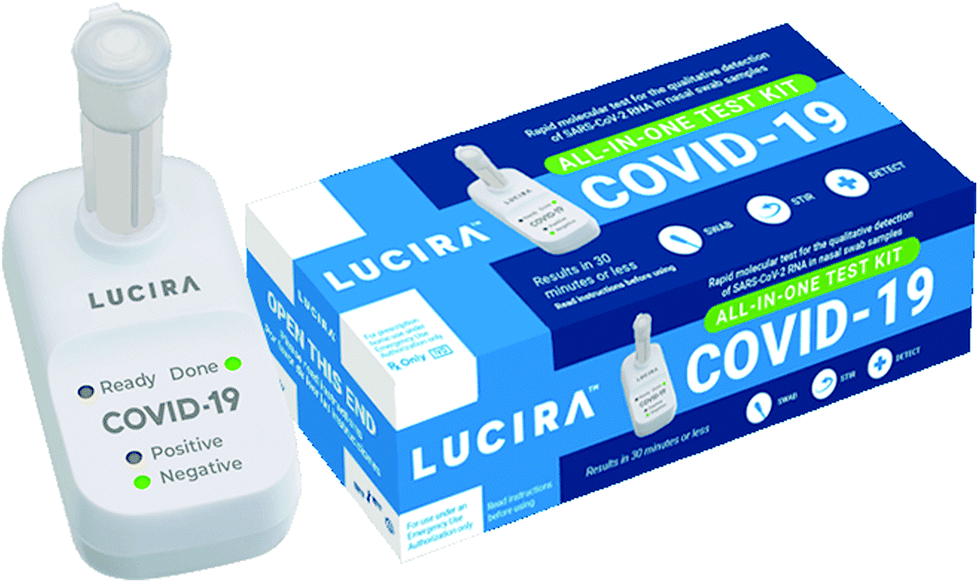
Developing Successful In Vitro Diagnostic Assays
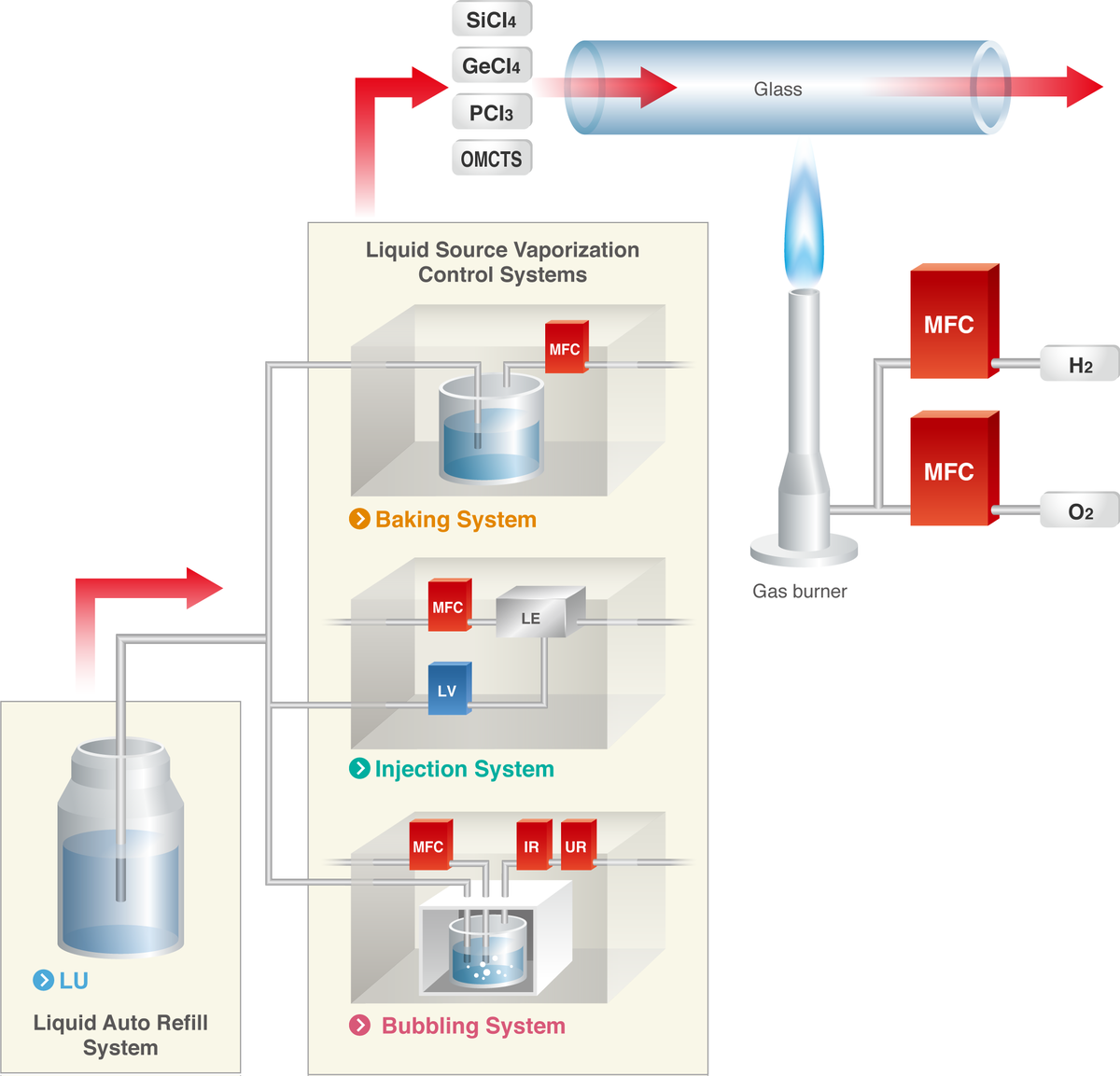
Optical Fiber Manufacturing Process - HORIBA

Pompes de diagnostic (IVD) In Vitro

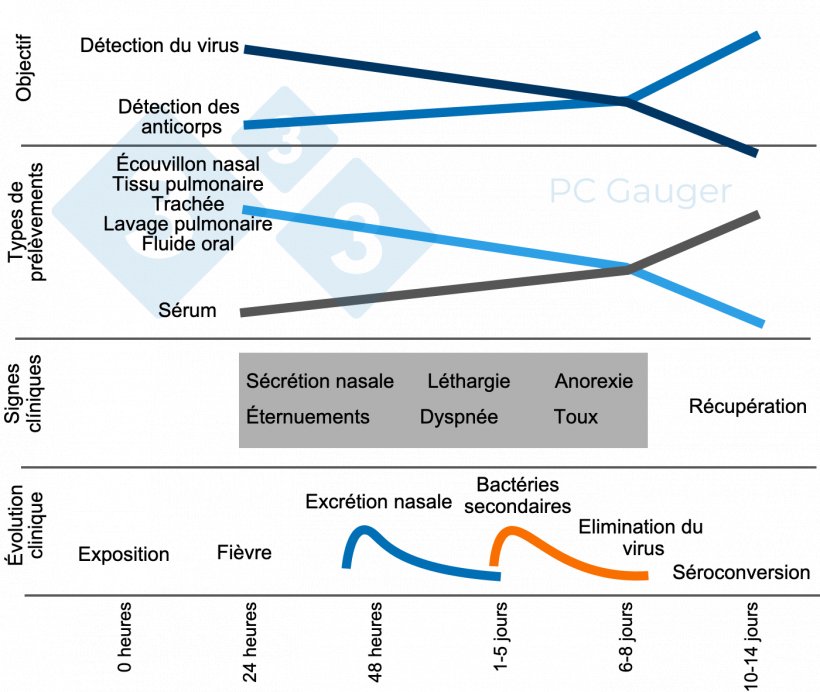
Schematic drawing of a simplified WHO diagnostic test kit for