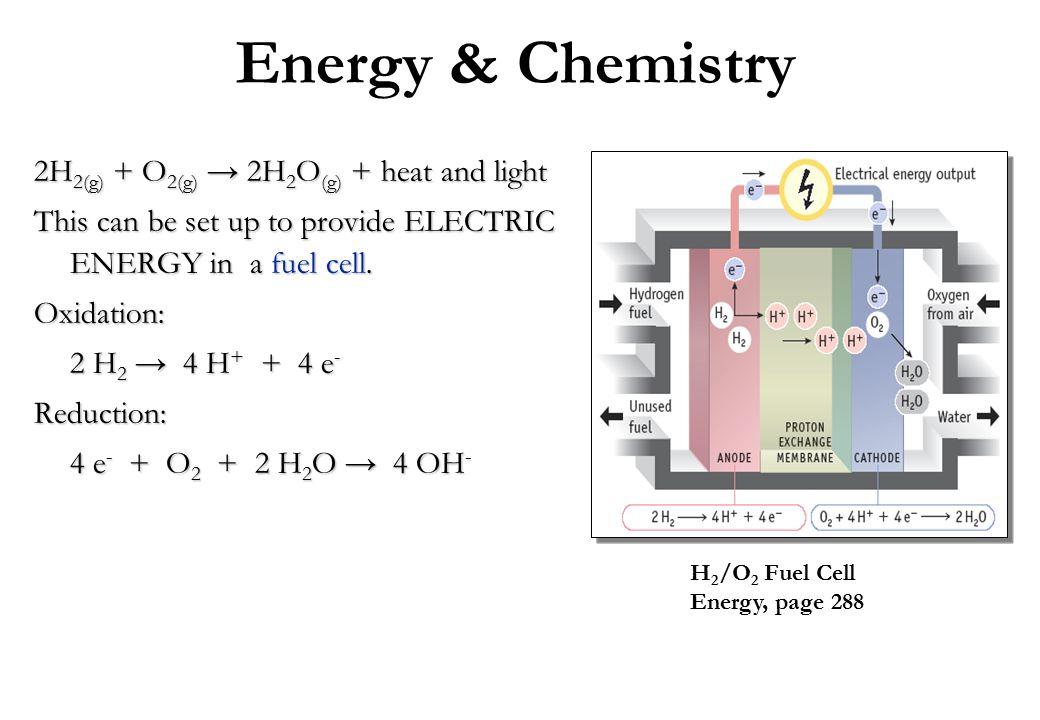
Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is
5 (595) · € 40.00 · En Stock

Heat of reaction . CO(g) + 1/O2(g) → CO2(g) constant V is-67.71 K 17℃ The heat of reaction constant P 17°C is (1-68 K a 2. 1S- (91

Compare Enthalpy Change of Formation of CO and CO2

1st PUC Chemistry Question Bank Chapter 6 Thermodynamics - KSEEB Solutions

Trioxane flame speed sensitivity to elementary reaction rate

Heat of reaction C_6H_{12}O_6(s) + 6O_2 (g) rightarrow 6CO_2(g) +6H_2O(v) constant pressure is -651 kcal 17°C. Calculate the heat of reaction constant volume 17°C.
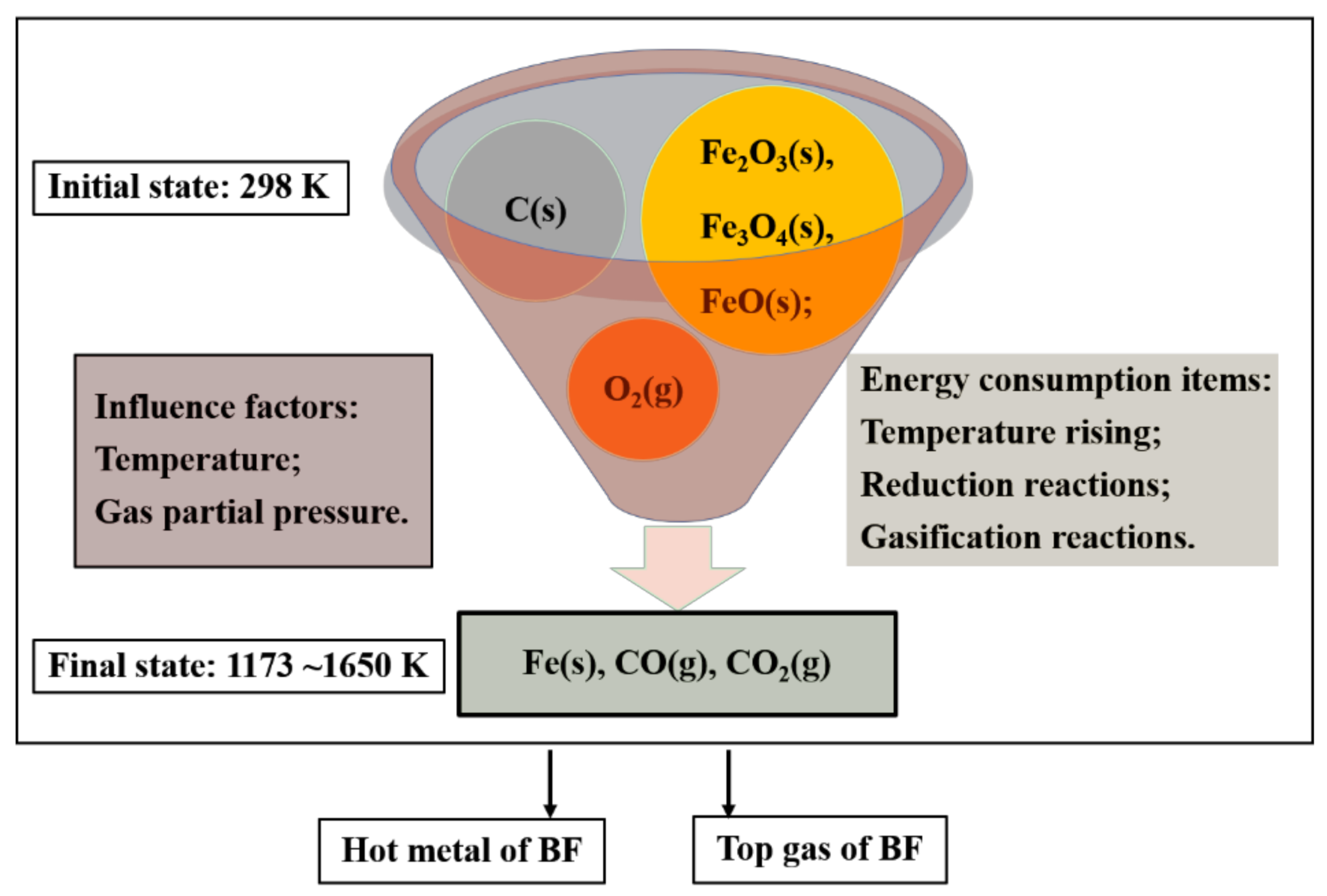
Energies, Free Full-Text

Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is

Effects of CO2 and N2 Dilution on the Combustion Characteristics of H2/CO Mixture in a Turbulent, Partially Premixed Burner

THERMOCHEMISTRY Thermodynamics - ppt download

A Study on CO2 Decomposition to CO and O2 by the Combination of Catalysis and Dielectric-Barrier Discharges at Low Temperatures and Ambient Pressure




)